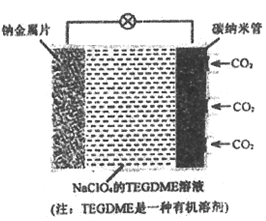
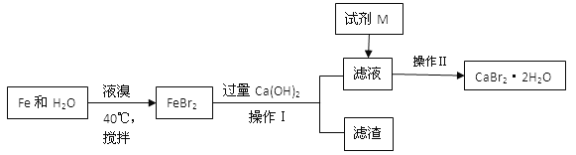
��Ŀ����
����Ŀ��Ϊ��̽����Һ������������Ũ�ȵĹ�ϵ��ij����С�����������ʵ�飺
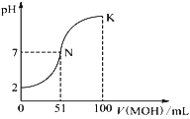
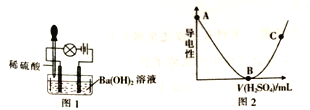
ȡһ������Ba(OH)2��Һ��������ʵ�飬���ձ��еμ�ϡ���ᡢװ����ͼ1.�����Һ����ǿ����ͼ2��ʾ��
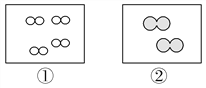
��1��������ɷ��ࡢ���η�Ϊ���Σ���Na2CO3������ʽ�Σ���NaHCO3������ʽ��[���ʽ̼��ͭCu2(OH)2CO3��]��NaHSO4����___________������ĸ����
A.���� B.������ C.��ʽ�� D.������
��2�����ձ��еμ�ϡ����Ĺ����У��۲쵽���ݱ仯����������Ϩ���������ɹ۲쵽��������_________��
��3��ͼ2�У�AB�α仯��ԭ����__________��BC�α仯��ԭ����_________��
��4��д��A��B�����з�����Ӧ�����ӷ���ʽ��_________��
���𰸡� AC �а�ɫ�������� ��Ba(OH)2��Һ�еμ�ϡ���ᣬBa2+��OH����Ũ����С�������Լ��� ����H2SO4����������࣬c(H+)��c(SO42��)����������ǿ Ba2++2OH��+2H++SO42����BaSO4��+2H2O
���������������������ͬ�����£���Һ������Ũ��Խ����Һ�ĵ�����Խǿ�����ݾ�Խ��������ͨ�����ݷ���ǿ����̽����Һ������������Ũ�ȵĹ�ϵ��
��1��NaHSO4��NaHCO3�������������κ���ʽ�Σ���AC��
��2������������������Һ��Ӧ�������ᱵ������ˮ����Һ������Ũ����С���������������Һ������Ũ��������������ձ��еμ�ϡ����Ĺ����У��۲쵽���ݱ仯����������Ϩ���������ɹ۲쵽���������а�ɫ����������
��3��ͼ2�У�AB�α仯��ԭ������Ba(OH)2��Һ�еμ�ϡ���ᣬBa2+��OH����Ũ����С�������Լ�����BC�α仯��ԭ��������H2SO4����������࣬c(H+)��c(SO42��)����������ǿ��
��4��A��B�����з�����Ӧ�����ӷ���ʽΪBa2++2OH��+2H++SO42����BaSO4��+2H2O��

 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�