��Ŀ����
3����1��ͨ����˵������ϳɲ�����ָ���ϡ��ϳ���ά�ͺϳ�����2�����ϵ���Ҫ�ɷ��Ǻϳ���֬�������������Ҫ����ijЩ�ض���;�����Ӽ���������������Ե����ܼ�����ֹ�����ϻ��ķ��ϻ����ȣ�
��3��������Ʒ�����������������Ź㷺��Ӧ�ã���������Ʒ�ڸ����ǵ������
�������ͬʱ��Ҳ���������ص�Σ�����������⣮
���ɾ�����ϩ�����������������Ⱦ�а�ɫ��Ⱦ��
�����Ϸ������Σ����A��
a�����Էֽ⣬�ƻ������ṹ��Ӱ��ֲ������ b����Ⱦ����
c��Σ��������������� d����ɺ����¼�
e���ƻ�������������Ⱦ����ˮ
A��abcde B��abcd C��bcd D��ae��
���� ��1������ϳɲ�����ָ�����ϡ��ϳ���ά���ϳ���
��2���������ɺϳ���֬�Ƴɵģ������г��������ܼ������ϻ����ȣ�
��3���١���ɫ��Ⱦ����Ҫ��ָ��������û�еõ����ƹ����ʹ����Ի�����ɵ���Ⱦ��
���þ۱���ϩ���۱�ϩ��������ϩ�ȸ߷��ӻ������Ƴɵĸ�������������Ʒ����Ȼ�������ڽ������������ɳ��л���������Ⱦ�������ڶѻ����ƻ���������Ⱦ����ˮ��Σ��������������棬��ɺ����¼��ȣ�
��� �⣺��1�����ϡ��ϳ���ά���ϳ���������ϳɲ��ϣ��ʴ�Ϊ�����ϣ��ϳ���ά���ϳ���
��2���������ɺϳ���֬�Ƴɵģ�Ϊ��������ܣ������г��������ܼ������ϻ����ȣ��ʴ�Ϊ���ϳ���֬�����ܼ������ϻ�����
��3�����������������Ⱦ�а�ɫ��Ⱦ���ʴ�Ϊ����ɫ��Ⱦ��
���þ۱���ϩ���۱�ϩ��������ϩ�ȸ߷��ӻ������Ƴɵĸ�������������Ʒ����Ȼ�������ڽ������������ɳ��л���������Ⱦ�������ڶѻ����ƻ���������Ⱦ����ˮ��Σ��������������棬��ɺ����¼��ȵȣ�����abcde���������Ϸ������Σ�����ʴ�Ϊ��A��
���� ���⿼��߷��ӻ���������Ժ��ȹ������ϡ�������Ʒ�������Σ������Ŀ�ѶȲ���ע��������Ʒ����Ȼ�������ڽ�������ص㣮

| A�� | 28 g���������е�ԭ����ĿΪ2 NA | |
| B�� | 4 g�����Ʊ�ɸ�����ʱʧȥ�ĵ�����ĿΪ0.1 NA | |
| C�� | 1 mol�����������NA��������ӵ�����֮����� | |
| D�� | ��״���£�22.4 L�������ϩ����������ķ�����ΪNA |
| A�� | ��صĵ��ҺΪ������Һ������ΪNi2O3������ΪFe | |
| B�� | ��طŵ�ʱ��������ӦΪFe+2OH--2e-=Fe��OH��2 | |
| C�� | ��س��ʱ��������ӦΪ2Ni��OH��2+2OH--2e-=Ni2O3+3H2O | |
| D�� | ��س������У�����������Һ��pH���� |
| A�� | ����е�NaOH��Һ�еμ�FeCl3������Һ�Ʊ�Fe��OH��3���� | |
| B�� | �����˶��ǽ����������е��˶���ʽ�����Ծݴ˰ѽ�������Һ������Һ���� | |
| C�� | �������е�ɣ�����������ɢϵ���ǵ����Ե� | |
| D�� | �������Ӻ�С����������Ĥ |
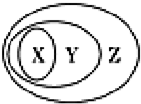 ����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в�����ȷ���ǣ�������
����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в�����ȷ���ǣ�������| X | Y | Z | |
| A | ǿ����� | ����� | ������ |
| B | ���� | ��ɢϵ | ����� |
| C | �û���Ӧ | ������ԭ��Ӧ | ���ӷ�Ӧ |
| D | ���������� | ���������� | ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �����������������ⸯʴʱ��������������������Fe-3e-=Fe3+ | |
| B�� | SO2����ͨ��Fe2��SO4��3��Һ�У�SO2+2H2O+2Fe3+=2Fe2++4H++SO42- | |
| C�� | ��ͭ���缫���CuSO4��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$ 2Cu+O2��+4H+ | |
| D�� | ������Һ�еμ�Ba��OH��2��Һ��SO42-��ȫ������Al3++Ba2++SO42-+3OH-�TBaSO4��+Al��OH��3�� |
 ���ײ���TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�ã��Ʊ�����TiO2�ķ���֮һ��TiCl4�ڼ���������ˮ������TiO2•xH2O���������ˡ�ˮϴ��ȥ���е�Cl-���پ���ɡ����ճ�ȥˮ�֣����õ�����TiO2��
���ײ���TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�ã��Ʊ�����TiO2�ķ���֮һ��TiCl4�ڼ���������ˮ������TiO2•xH2O���������ˡ�ˮϴ��ȥ���е�Cl-���پ���ɡ����ճ�ȥˮ�֣����õ�����TiO2��