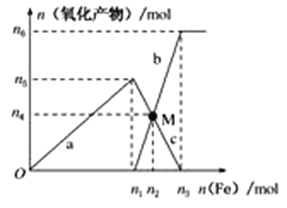
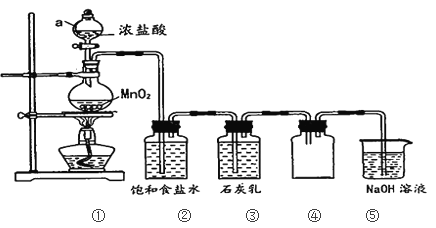
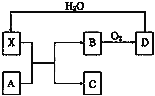
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷Σœ¬Ν–Ζ¥”ΠΘΚ2CO(g)+O2(g)=2CO2(g) ΠΛH=-566kJΓΛmol-1Θ§Na2O2(s)+CO2(g)=Na2CO3(s)+ ![]() O2(g) ΠΛH=-266kJΓΛmol-1Θ§ ‘ΜΊ¥πΘΚ
O2(g) ΠΛH=-266kJΓΛmol-1Θ§ ‘ΜΊ¥πΘΚ
(1)COΒΡ»Φ…’»»ΠΛH=_______________ΓΘ
(2)‘Ύ¥ΏΜ·ΦΝΉς”Οœ¬Θ§“Μ―θΜ·ΧΦΩ…”κΙΐ―θΜ·ΡΤΖ¥”Π…ζ≥…ΙΧΧεΧΦΥαΡΤΘ§ΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚ________ΓΘ
(3)ΙΛ“ΒΖœΤχ÷–ΒΡCO2Ω…”ΟΦν“ΚΈϋ ’Θ§ΥυΖΔ…ζΒΡΖ¥”Π»γœ¬ΘΚCO2(g)+2NaOH(aq)=Na2CO3(aq)+H2O(l) ΠΛH=-a kJΓΛmol-1Θ§CO2(g)+NaOH(aq)=NaHCO3(aq) ΠΛH=-bkJΓΛmol-1Θ§‘ρΘΚ
ΔΌCO2(g)+H2O(l)+Na2CO3(aq)=2NaHCO3(aq)ΒΡΠΛH=________kJΓΛmol-1(”ΟΚ§aΓΔbΒΡ¥ζ ΐ Ϋ±μ Ψ)ΓΘ
ΔΎ±ξΩωœ¬Θ§11.2LCO2”κΉψΝΩΒΡNaOH»ή“Κ≥δΖ÷Ζ¥”ΠΚσΘ§Ζ≈≥ωΒΡ»»ΝΩΈΣ_______kJ(”ΟΚ§aΜρbΒΡ¥ζ ΐ Ϋ±μ Ψ)ΓΘ
(4)ΗυΨί“‘œ¬»ΐΗω»»Μ·―ßΖΫ≥Χ ΫΘΚ2H2S(g)+3O2(g)= 2SO2 (g) +2H2O (l) ΠΛH=-Q1kJΓΛmol-1Θ§2H2S(g)+O2(g)= 2S (s) +2H2O (l) ΠΛH=-Q2kJΓΛmol-1Θ§2H2S(g)+O2(g)=2 S (s) +2H2O (g) ΠΛH=-Q3kJΓΛmol-1Θ§≈–ΕœQ1ΓΔQ2ΓΔQ3ΒΡ¥σ–ΓΙΊœΒ «____ΓΘ
ΓΨ¥πΑΗΓΩ- 283kJΓΛmol-1 CO(g)+Na2O2(s)=Na2CO3(s) ΠΛH=-549kJΓΛmol-1 (a-2b)kJΓΛmol-1 ![]() kJ Q1ΘΨQ2ΘΨQ3
kJ Q1ΘΨQ2ΘΨQ3
ΓΨΫβΈωΓΩ
(1)»Φ…’»» «1molΩ…»ΦΈοΆξ»Ϊ»Φ…’…ζ≥…Έ»Ε®―θΜ·Έο ±Ζ≈≥ω»»ΝΩΘΜ
(2)“άΨίΗ«ΥΙΕ®¬…ΫαΚœ“―÷ΣΖΫ≥Χ ΫΦΤΥψΘΜ
(3)ΔΌ“άΨίΗ«ΥΙΕ®¬…ΫαΚœ“―÷ΣΖΫ≥Χ ΫΦΤΥψΘΜΔΎΗυΨί»»Μ·―ßΖΫ≥Χ ΫΘ§άϊ”ΟΈο÷ ΒΡΝΩ÷°±»Β»”Ύ»»ΝΩ±»ΦΤΥψΘΜ
(4)“άΨίλ ±δΒΡΚ§“εΚΆΖ¥”ΠΈο÷ ΒΡΨέΦ·Ή¥Χ§±δΜ·Θ§Ζ¥”ΠΒΡΫχ––≥ΧΕ»Ζ÷Έω≈–ΕœΓΘ
(1)2CO(g)+O2(g)=2CO2(g)ΓςH=-566kJ/molΘ§»Φ…’»» «1molΩ…»ΦΈοΆξ»Ϊ»Φ…’…ζ≥…Έ»Ε®―θΜ·Έο ±Ζ≈≥ω»»ΝΩΘ§‘ρ“Μ―θΜ·ΧΦΒΡ»Φ…’»»ΈΣΓςH=-283kJ/molΘ§Ι ¥πΑΗΈΣΘΚ-283kJ/molΘΜ
(2)“―÷ΣΔΌ2CO(g)+O2(g)=2CO2(g)ΓςH=-566kJ/molΘ§ΔΎNa2O2(s)+CO2(g)=Na2CO3(s)+ ![]() O2(g)ΓςH=-266kJ/molΘ§”…Η«ΥΙΕ®¬…ΘΚ
O2(g)ΓςH=-266kJ/molΘ§”…Η«ΥΙΕ®¬…ΘΚ![]() ΓΝΔΌ+ΔΎΒΟCO(g)+Na2O2(s)=Na2CO3(s)ΓςH=
ΓΝΔΌ+ΔΎΒΟCO(g)+Na2O2(s)=Na2CO3(s)ΓςH=![]() ΓΝ(-566)+(-266)=-549 kJ/molΘ§Ι ¥πΑΗΈΣΘΚCO(g)+Na2O2(s)=Na2CO3(s)ΓςH=-549 kJ/molΘΜ
ΓΝ(-566)+(-266)=-549 kJ/molΘ§Ι ¥πΑΗΈΣΘΚCO(g)+Na2O2(s)=Na2CO3(s)ΓςH=-549 kJ/molΘΜ
(3)ΔΌi.CO2(g)+2NaOH(aq)=Na2CO3(aq)+H2O(l)ΓςH=-a kJ/molΘ§ii.CO2(g)+NaOH(aq)=NaHCO3(aq)ΓςH=-b kJ/molΘ§”…Η«ΥΙΕ®¬…ΘΚiiΓΝ2-iΒΟ CO2(g)+H2O(l)+Na2CO3(aq)=2NaHCO3(aq)ΒΡΓςH=(a-2b)kJ/molΘ§Ι ¥πΑΗΈΣΘΚ(a-2b)kJ/molΘΜ
ΔΎCO2”κΉψΝΩΒΡNaOH»ή“Κ≥δΖ÷Ζ¥”ΠΘ§ΤδΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚCO2(g)+2NaOH(aq)=Na2CO3(aq)+H2O(l)ΓςH=-a kJ/molΘ§±ξΩωœ¬Θ§11.2L CO2ΒΡΈο÷ ΒΡΝΩΈΣn(CO2)=![]() =0.5molΘ§‘ρΖ≈≥ωΒΡ»»ΝΩΈΣ0.5molΓΝa kJ/mol=
=0.5molΘ§‘ρΖ≈≥ωΒΡ»»ΝΩΈΣ0.5molΓΝa kJ/mol=![]() kJΘ§Ι ¥πΑΗΈΣΘΚ
kJΘ§Ι ¥πΑΗΈΣΘΚ![]() kJΘΜ
kJΘΜ
(4)ΔΌ2H2S(g)+3O2(g)=2SO2(g)+2H2O(1) ΓςH=-Q1kJ/molΘ§ΔΎ2H2S(g)+O2(g)=2S(g)+2H2O(1) ΓςH=-Q2kJ/molΘ§Δέ2H2S(g)+O2(g)=2S(g)+2H2O(g) ΓςH=-Q3kJ/molΘ§Ζ¥”ΠΔΎΔέœύ±»Θ§…ζ≥…ΤχΧ§Υ°ΚΆ…ζ≥…“ΚΧ§Υ°œύ±»Θ§…ζ≥…“ΚΧ§Υ°Ζ≈≥ωΒΡ»»ΝΩΕύΘ§Υυ“‘Q2ΘΨQ3ΘΜΖ¥”ΠΔΌΔΎœύ±»Θ§ΔΎΖ¥”Π…ζ≥…ΒΞ÷ ΝρΘ§ΒΞ÷ ΝρΉΣΜ·ΈΣΕΰ―θΜ·Νρ ±ΜαΖ≈≥ω»»ΝΩΘ§Υυ“‘QlΘΨQ2ΘΜΉέ…œΥυ ωQ1ΓΔQ2ΓΔQ3»ΐ’Ώ¥σ–ΓΙΊœΒΈΣΘΚQlΘΨQ2ΘΨQ3Θ§Ι ¥πΑΗΈΣΘΚQlΘΨQ2ΘΨQ3ΓΘ