��Ŀ����
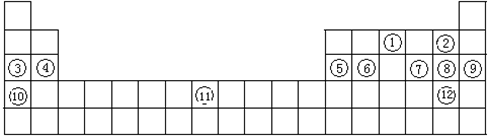
��9�֣��±���Ԫ�����ڱ���һ���֣������ݸñ��ش����⡣
| �� ���� | IA |
| 0 | |||||
| 1 | H | IIA | IIIA | IVA | VA | VIA | VIIA | He |
| 2 | Li | Be | B | C | N | O | F | Ne |
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar |
��1������˵����ȷ���� ��
A�������ڰ뵼����ϵ�Ԫ����̼
B��PH3���ȶ��Ա�H2Sǿ
C����VA��Ԫ�ص�����������Ӧ��ˮ���ﻯѧʽ��ΪH3RO4
D��H2SiO3�����Ա�H3PO4��
E��NaOH�ļ��Ա�Mg(OH)2ǿ
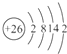
��2����2����VIIA��Ԫ�ص�ԭ�ӽṹʾ��ͼ�� _______________ ����Ԫ����ͬ����IA��Ԫ����ɵĻ����������� ��_______________
��3����3����Ԫ�ص�����������Ӧˮ�����У�������ǿ���� _______________���ѧʽ����ͬ����������ǿ���� _______________ ���������Ե��� _______________ ��
��9�֣�
��1��DE![]() ��3�֣�
��3�֣�
��2��
��2�֣� ���ӻ����� ��1�֣�
��3��HClO4 NaOH Al(OH)3 ��3�֣�
����:��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
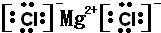
 NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ��
NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ�� ��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����
��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����