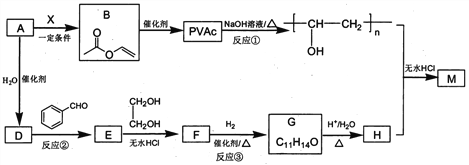
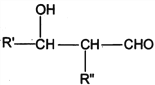
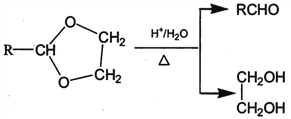
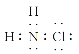��Ŀ����
����Ŀ����A��B��C��D���ֶ�����Ԫ�أ����ǵ�ԭ��������A��D����������֪A��Bԭ������ͬ�ĵ��Ӳ�������A��L���������K���������������Cȼ��ʱ���ֻ�ɫ���棬C�ĵ����ڵ�ȼ��������B�ĵ��ʳ�ַ�Ӧ�����Եõ���D������ɫ��ͬ�ĵ���ɫ��̬������Ը������������ش�
��1��Ԫ������: A ____________ B ____________ C ____________ D ____________��
��2��д��AB2�ĵ���ʽΪ___________��
��3���õ���ʽ��ʾ������C2D���γɹ���___________________________________��
���𰸡�̼ �� �� �� ![]()
![]()
��������
A��Bԭ������ͬ�ĵ��Ӳ�����˵��A��Bλ��ͬ���ڣ�A��L���������K������������������ݺ�������Ų����ɣ��Ƴ�AΪC��C��ȼ��ʱ���ֻ�ɫ���棬�Ƴ�CԪ��ΪNa��Na��B�ĵ��ʳ�ַ�Ӧ�����Եõ�����ɫ��̬������û�����ΪNa2O2����BΪO��D���ʵ���ɫΪ����ɫ����DΪS���ݴ˷�����
A��Bԭ������ͬ�ĵ��Ӳ�����˵��A��Bλ��ͬ���ڣ�A��L���������K������������������ݺ�������Ų����ɣ��Ƴ�AΪC��C��ȼ��ʱ���ֻ�ɫ���棬�Ƴ�CԪ��ΪNa��Na��B�ĵ��ʳ�ַ�Ӧ�����Եõ�����ɫ��̬������û�����ΪNa2O2����BΪO��D���ʵ���ɫΪ����ɫ����DΪS��
(1)��������������AΪ̼��BΪ����CΪ�ƣ�DΪ��
(2)�γ�AB2ΪCO2����ṹʽΪO=C=O�� ��CO2�ĵ���ʽΪ![]() ��
��
(3)C2DΪNa2S��Na2S�������ӻ��������Na����S2����ɣ����õ���ʽ��ʾNa2S�γɵĹ��̣�![]() ��
��

����Ŀ��NiCl2�ǻ����ϳ�������Ҫ����Դ����ʵ������ģ�ҵ���Խ���������(��Fe��Al������)Ϊԭ������NiCl2�Ĺ����������£�
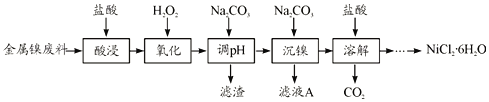
�±��г�����ؽ������������������������pH
�������� | Fe(OH)3 | Fe(OH)2 | Al(OH)3 | Ni(OH)2 |
��ʼ������pH | 2.1 | 6.5 | 3.7 | 7.1 |
������ȫ��pH | 3.3 | 9.7 | 4.7 | 9.2 |
(1)Ϊ�������Ԫ�صĽ����ʣ����������ʱ�ɲ�ȡ�Ĵ�ʩ��__________(дһ������)��
(2)����H2O2ʱ������Ҫ��Ӧ�����ӷ���ʽΪ__________��
(3)����pH��ʱ��������ҺpH�ķ�ΧΪ__________��
(4)�������������У�����ҺA��c(Ni2��)=1.0mol/L����ʹ100mL����Һ�е�Ni2��������ȫ�ۼ���Һ��c(Ni2��)��1.0��10-5�ݣ�������������ƽ��ȡNa2CO3�������������Ϊ_____g��(��֪Ksp(NiCO3)=6.5��10-6��������Һ����ı仯)
(5)����������Һ�õ�NiCl2��6H2O��ʵ�������������Ϊ______�����ˡ�ϴ�ӡ����