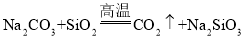��Ŀ����
����Ŀ����֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣
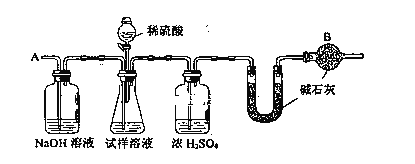
��Ҫʵ�鲽�����£�
�� ��ͼ��װ������������װ�õ�������
�� ��a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�� ����ʢ�м�ʯ�ҵ�U�ܵ��������õ�b g
�� �ӷ�Һ©������6mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ
�� �ӵ���A����������һ�����Ŀ���
�� �ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c g
�� �ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊd g
��ش������й����⣺
(1)����������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��________________��
(2)װ���и����B��������______________________________________________��
(3)�������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ��_________��(ѡ��ƫ�ߡ�ƫ�ͻ�)��
(4)����ݵ�Ŀ����__________________________________________��
(5)����ߵ�Ŀ����__________________________________________��
(6)�����д�������������ļ���ʽΪ__________________________��
(7)д��������ϡ���ᷴӦ�����ӷ���ʽΪ________________________��
���𰸡���Ʒ�أ������� ��ֹ�����е�CO2��ˮ������U���� ƫ�� �ѷ�Ӧ������CO2ȫ������U�ι��� �жϷ�Ӧ������CO2�Ƿ�ȫ���ų�������U�ι��еļ�ʯ������ ![]() ��100% CO32-��2H+= CO2��+H2O
��100% CO32-��2H+= CO2��+H2O
��������
(1)������ƽ����ʱ�������������ԭ���ǣ�
(2)���ڿ�����Ҳ�ж�����̼��ˮ�֣������B�����þ��Dz������ǽ���ģ�
(3)��������Ļӷ��Կ��ǣ�
(4)���ڷ�Ӧ��������ƿ�д����ж�����̼������һ�����Ŀ�������Ϊ���������ǵģ�
(5)����Ϊ�˽�������̼ȫ���Ϲ�ȥ��
(6)����U�ܵ��������������������ɵĶ�����̼�����������ݶ�����̼���������̼���Ƶ�����������̼���Ƶ�����������Ʒ�������ɡ�
(7) ���������ᷴӦ���������ơ�������̼��ˮ�������������Ϊǿ����ʣ�д��ѧʽ��
(1)���ڳ���ʱ�������룬����ƫ˵����Ʒ�أ������
(2)U���еļ�ʯ����Ϊ�����շ�Ӧ���ɵĶ�����̼����������Ҳ���ڶ�����̼�������B�����þ��Ƿ�ֹ�����еĶ�����̼��ˮ�ֽ���U�ܣ��Խ��������
(3)����������лӷ��ԣ�Ҳ�����Ŷ�����̼����U�ܣ�������Ϊ�Ƕ�����̼�����Զ�����̼������ƫ�������̼���Ƶ�����Ҳ��ƫ��ģ����Խ����ƫ�ߣ�
(4)���ڷ�Ӧ��������ƿ�д����ж�����̼������һ�����Ŀ������ǽ������Ķ�����̼��ȫ����U�ܣ�
(5)ֱ��U�ܵ������������䣬˵��������̼�Ѿ���ȫ���ŵ�U���У�����ߵ�Ŀ���ǣ��жϷ�Ӧ������CO2�Ƿ�ȫ���ų�������U�ι��еļ�ʯ�����գ�
(6)����Ҫ̼���Ƶ�����Ϊm��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
m db
�б���ʽ��ã�m=![]() �����������д�������������ļ���ʽ=
�����������д�������������ļ���ʽ=![]() ��100%��
��100%��
(7)���������ᷴӦ���������ơ�������̼��ˮ�������������Ϊǿ����ʣ�д��ѧʽ�������ӷ���ʽΪCO32-��2H+= CO2��+H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����������Ϊһ�ֳ���ҩ�����н�����ʹ��Ч����ʵ�����Ʊ���������ʱ�������ñ�������������������ȡ���÷�Ӧ���ȣ�
�ܶȣ�g/mL�� | ��Է������� | ��ɫ��״̬ | �ܽ��� | |
���� | 1.04 | 93 | ��ɫ��״Һ�� | ����ˮ�� �������Ҵ������� |
������ | 1.08 | 102 | ��ɫ��Һ�� | ��ˮ������Ӧ�������� |
�������� | 135 | ��ɫƬ״���壬 �۵� 114�� | ��������ˮ���������� ˮ���Ҵ������� |
ʵ�鲽��
��ȡ5.00 mL����������100 mL��ƿ�У�����20 mLˮ������ҡ�·�������6.00 mL ��������������ȡ����н������������ò���������״�����飬�ٳ�ֽ��裻
�ڷ�Ӧ��ȫ��ʱ�ѷ�Ӧ�����ת�Ƶ��ձ��У���ȴ���ˣ�ϴ�ӣ��ô������������壻
�۽��ֲ�Ʒת����150 mL�ձ��У���������ˮ���Ƴ� 80 ��ı�����Һ���ټ������20%��ˮ������Ӱ����̿�������½���Һ���3��5 min��_______����ȴ�ᾧ�����ˡ�ϴ�ӡ����ɵ�����������Ʒ6.2 g��
��ش�
��1����Ӧ�¶ȹ��ᵼ�±����ӷ������в����ɿ��Ʒ�Ӧ�¶ȣ���ֹ��Ӧ�¶����߹������______��
A ��20 mLˮ B ��ҡ�·�������6.0 mL������
C ����������״������ D ��ֽ���
��2���ڲ�����жԴֲ�Ʒ����ϴ����Ҫ�õ����²�����
a ����ϴ�Ӽ�����û���壻b ϴ�Ӽ�����ͨ����c ��Сˮ��ͷ��d ����ˮ��ͷ�� e �ظ� 2��3 �Ρ�
������ϲ���������ȷ������__________��
��3������ۼ������ 20%��ˮ��Ŀ����________�����ߴ��IJ���������_________��
��4������˵������ȷ����______��
A ����̿������ɫ���ʣ�������߲���
B ��ȴʱ��������ȴ�ȱ�ˮԡ��ȴ���õ������������ڳ���
C ��������ͼװ�ã�Ϊ��ֹ����������ʱ���ȹر�ˮ��ͷ�������a
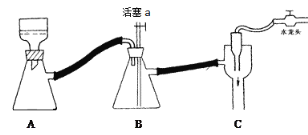
D ��Ʒ�ɷ��ڱ�����������ˮԡ��ɣ���ɺ��ͨ��������Ʒ�۵��жϲ�Ʒ����
��5����ʵ��IJ�����___________��