��Ŀ����
�����ɵ�������NaHCO3��KHCO3��ɵĻ����a g����100 mL���ᷴӦ��(�����漰������������Ա�״���ƣ����ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
(1)�û������NaHCO3��KHCO3�����ʵ���֮��Ϊ ��
(2)��̼������������ǡ����ȫ��Ӧ����������HCl�����ʵ���Ϊ mol��
(3)����������������CO2�����Ϊ L��
(4)�����Ӧ��̼��������ʣ�࣬���������Ҫ��������CO2�����������Ҫ֪�� ��
(5)��NaHCO3��KHCO3�����Ե�������ϣ���a g����������������������ȫ��Ӧʱ����CO2�����[V(CO2)]��Χ�� ��
(1)100��84(��25��21)
(2)  mol����
mol����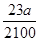 mol��
mol��
(3)  L
L
(4)��������ʵ���Ũ��(����������Ҳ��)
(5) 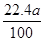 L<V(CO2)<
L<V(CO2)< 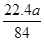 L
L
����

��ϰ��ϵ�д�
�����Ŀ
��Ũ��������һ�����ʵ���Ũ�ȵ�������Һ�����в��������������ҺŨ��ƫ�ߵ��ǣ� ��
| A���ܽ�����Һδ��ȴ�����¾�ת��������ƿ�� |
| B��������תҡ�Ⱥ�Һ����ڿ̶��ߣ��ټ�����ˮ��Һ����͵�ǡ����̶�����ƽ |
| C��ϴ���ձ��Ͳ���������Һת��������ƿ�� |
| D������ʱ���۾����ӿ̶��� |

 2CO��2H2��CH4��H2O
2CO��2H2��CH4��H2O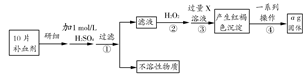

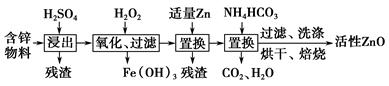

 Al2��SO4��3��
Al2��SO4��3��