��Ŀ����
��ҵ���ú�п����(��FeO��CuO������)���Ƶû���ZnO���������£�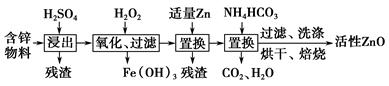
(1)���������У������õ���60%H2SO4(1.5 g��cm��3)����������H2SO4 100 mL��Ҫ18.4 mol��L��1��ŨH2SO4________ mL(����һλС��)��
(2)����������H2O2����Fe(OH)3�������֣�û��Cu(OH)2�������֣�����Һ��c(Fe3��)��2.6��10��18 mol��L��1������Һ��c(Cu2��)��ȡֵ��Χ��________mol��L��1��(��֪Ksp[Fe(OH)3]��2.6��10��39��
Ksp[Cu(OH)2]��2.2��10��20)
(3)����NH4HCO3�����ɵij�������̬��ΪZna(OH)b(CO3)c(a��b��cΪ������)�����ּ�ʽ̼��пA��B�Ļ���A��a��5��b��6�������ɼ�ʽ̼��пA�Ļ�ѧ����ʽΪ__________________________________________________��
(4)ȡϴ�ӡ���ɺ�ļ�ʽ̼��пA��B�Ļ����49.70 g�������ʵ���Ϊ0.10 mol�����±�����ȫ�ֽ�õ�37.26 g ZnO��3.584 L CO2(��״����)��ˮ��ͨ�����������ʽ̼��пB�Ļ�ѧʽ��
(1)49.9(50.0Ҳ����) (2)��2.2��10��6
(3)5ZnSO4��10NH4HCO3=Zn5(OH)6(CO3)2����5(NH4)2SO4��8CO2����2H2O
(4)������0.1 mol�������ȫ�ֽ�õ�ZnO��CO2��H2O�����ʵ����ֱ�Ϊ0.46 mol��0.16 mol��0.3 mol
��֪1 mol�������ƽ����4.6 mol Zn��1.6 mol C��6 mol H����֪1 mol A�к�HΪ6 mol����CΪ2 mol����1 mol B�к�HΪ6 mol����CΪ1 mol������B�Ļ�ѧʽ���Ա�ʾΪZnx(OH)6CO3���ɻ��ϼ۴�����Ϊ��ó�x��4����B�Ļ�ѧʽΪZn4(OH)6CO3(���������ⷨ����)
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ijУ��ѧС��ѧ�����С�������Է��������IJⶨ����ʵ�顣�������£����������ݻ�����ȵ���ƿ�ռ����壬�����ռ����������ƿ���������ݼ��±�(�ѻ���ɱ�״���µ���ֵ)��
| ���� | ��ƿ�������������(g) |
| A | 48.4082 |
| B | 48.4082 |
| C | 48.4082 |
| D | 48.4342 |
| E | 48.8762 |
��֪��״���£���ƿ���ݻ�Ϊ0.293 L����ƿ�Ϳ�����������Ϊ48.4212 g��������ƽ����Է�������Ϊ29��A��B��C��D��E����ѧ���������塣
(1)�������������У��ܹ�ʹƷ����Һ��ɫ����(д��ѧʽ)________��
(2)D����Է���������________��
(3)�ڱ�״���£�11.2 L D�����к��й��õ��ӶԵ���ĿΪ________��
(4)A��B��C���ܵĻ�ѧʽ��________��
ͭ����Ͻ�����������ʹ�õĽ������ϡ�
(1)����ͭ��ȡ�������ַ�ʽ�ѻ�(����)

(2)��1��Cu2O������(�ṹ����ͼ��ʾ)��Cuԭ����λ��Ϊ__________��
(3)��ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�
�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ____________��
�ڵ�����SO42���Ŀռ乹��Ϊ________��H2O��Oԭ�ӵ��ӻ�����Ϊ________��
��ij��ȤС���ȡ2.500 g�������壬������ʹ��ʧˮ����ȷ�ⶨ��ͬ�¶���ʣ�������������õ���ͼ��ʾ��ʵ����ʾ��ͼ������˵����ȷ����(����)
| A������ӳ�������105 ��Ĺ�����ֻ��������� |
| B�������������γ���λ����4��ˮ����ͬʱʧȥ |
| C��120 ��ʱ��ʣ�����Ļ�ѧʽ��CuSO4��H2O |
| D������������ʧˮʱ���˷�����������С��ͬ�������е�ˮ���ӿ��Է�Ϊ3�� |
 MnFe2O4��x��O2����
MnFe2O4��x��O2���� MnFe2O4��xH2��
MnFe2O4��xH2��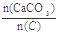 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ�� Ϊ��ֵ��
Ϊ��ֵ��