��Ŀ����
����Ŀ��ijʵ��С��ͬѧ��̽��SO2�����ʣ����ⶨ������SO2�ĺ����������������ʵ��װ�ã���ͼ�����������̽�������ش����⣺
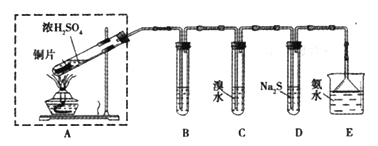
��1��װ��E�еİ�ˮ���������������SO2��������Ӧ�����ӷ���ʽ��____________��Eװ����ʹ�õ�����©����Ŀ����________________��
��2��װ��B���ڼ���SO2��Ư���ԣ�������ʢ�Լ�Ϊ____________________��װ��D���ڼ���SO2��______________���ʣ�
��3��װ��C�з�����Ӧ�����ӷ���ʽΪ_______________��
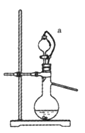
��4�������������������Ʒ�Ӧ��ȡ��������װ����ͼ��ʾ������a���ܵ�������_______��д����ƿ�з�Ӧ�Ļ�ѧ����ʽ ___________________��
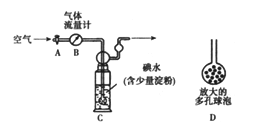
��5�������������·�������ͼ���ⶨ������SO2�����������������������ԭ�����壩��
����1:
�� ϴ��ƿC����Һ��ɫ��ʧ����û�м�ʱ�رջ���A�����õ�SO2����______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
������:
![]()
�� ʵ������ͨ���Ŀ��������Ϊ33.6L���ѻ���ɱ�״�������������ù�������Ϊ0.233g����ͨ������ȷ���ÿ����ж������������ٷֺ���____________��
���𰸡� SO2+2NH3��H2O=2NH4++SO32-+H2O ������ Ʒ����Һ ������ 2H2O+SO2+Br2=4H++SO42-+2Br- ʹ©���е�Һ����˳������ Na2SO3+H2SO4=Na2SO4+ SO2+H2O ƫ�� 0.067%
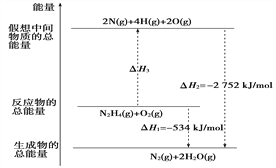
����������1�����������ж�����ֱ���ſգ��Ҷ���������������������ܺͼ���Һ��Ӧ���������κ�ˮ�������ð�ˮ���ն����������ӷ���ʽΪ��SO2+2NH3��H2O=2NH4++SO32-+H2O�����ڶ����������弫�����ڰ�ˮ������װ����ѹǿ���罵�ͣ�������ѹѹ��Һ����룬�������������õ��۵�©������Բ�β�������ϴ��л������ã��ܷ�ֹ��������2��װ��B���ڼ���SO2��Ư���ԣ�SO2����ijЩ��ɫ������Ʒ�����γ���ɫ�����ʣ����SO2��Ư���ԣ���װ��B��Ʒ����Һ���飬��װ��D�з�����Ӧ��SO2+2H2S=3S��+H2O����Ӧ��SO2�������������������ԣ�H2S�ǻ�ԭ�������ֻ�ԭ�ԣ���3����װ��C��SO2����ˮ������Ӧ��2H2O+SO2+Br2=4H++SO42-+2Br-�� ��4��a�������ӷ�Һ©����������ƿ����������ƽ���Һ©����������ƿ���ѹǿ��ʹҺ��˳�����£����з�Ӧ�ķ���ʽΪNa2SO3+H2SO4=Na2SO4+ SO2��+H2O����5����ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����ͨ��β��������������SO2����ƫ�ͣ���0.233g���ᱵ�����ʵ���Ϊ0.233g��233g/mol=0.001mol��������Ԫ���غ㣬��֪n��SO2��=n��H2SO4��=n��BaSO4��=0.01mol���ʶ�����������Ϊ0.01mol��22.4L/mol=0.0224L������������������Ϊ0.0224L/33.6L��100%��0.067%��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�