��Ŀ����
����Ŀ����1�������з���Ҫ��������ĸ������Ӧλ��
A��O2��O3 B.![]() C��
C��![]() C C��CH3CH2CH2CH3��
C C��CH3CH2CH2CH3��![]()
D�� E��CH3CH2CH2CH3��
E��CH3CH2CH2CH3��![]()
��___________����������Ϊͬλ�ء���__________���������ʻ�Ϊͬ�������塣
��___________��������������ͬϵ���___________�������ʻ�Ϊͬ���칹�塣
��___________����������ͬһ���ʡ�
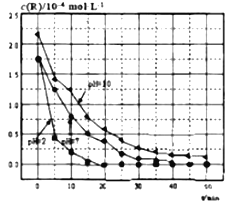
��2��һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g)![]() xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ__________________��XΪ________������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��________ 0.8mol/L������ڣ�С�ڻ���ڡ�����
xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ__________________��XΪ________������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��________ 0.8mol/L������ڣ�С�ڻ���ڡ�����
���𰸡� B A C E D 0.2mol/(L.min) 2 С��
����������1�����顰��ͬ������ͬλ������������ͬ����������ͬ��ͬ��Ԫ�ز�ͬ���أ���˷���Ҫ�����B����B��ȷ������ͬ��Ԫ����ɲ�ͬ�ṹ�ĵ��ʻ�Ϊͬ�������壬��A��ȷ����ͬϵ���ǽṹ���ƣ��ڷ�����������һ�������ɸ�CH2��ԭ���ŵĻ�����������ͬϵ�����C���ܷ���ʽ��ͬ�ṹ��ͬ�Ļ����ﻥ��Ϊͬ���칹�壬��E��ȷ��������ͬһ�����ʵ���D����2�����黯ѧ��Ӧ���ʵļ��㣬����A�����ʵ���Ϊ��3��1.8��mol=1.2mol��������B�����ʵ���Ϊ1.2/3mol=0.4mol�����ݻ�ѧ��Ӧ���ʵ���ѧ����ʽ��v(B)=0.4/(2��1)mol/(L��min)=0.2 mol/(L��min)��1min����C�����ʵ���Ϊ1.2x/3mol=0.4��2�����x=2�����跴Ӧ���ʲ��䣬��ʱ������C��Ũ��Ϊ0.8mol��L��1�������ŷ�Ӧ���У���Ӧ��Ũ�Ƚ��ͣ���ѧ��Ӧ���ʼ��������2min�ﵽƽ��ʱ������C��Ũ��ӦС��0.8mol��L��1��

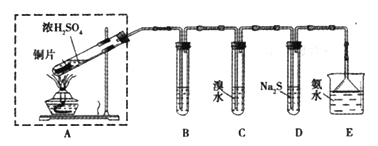
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�����Ŀ��ij��ȤС�����÷Ͼɾ���������Ʊ����������������£�
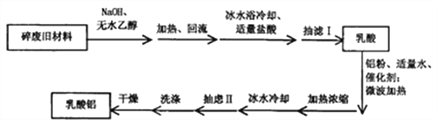
��֪���ٷ�Ӧԭ����
���᳣����Ϊ������ˮ���Ҵ����ܼ���Һ�壻������Ϊ��ɫ���ɫ��ĩ״���壬����ˮ���������Ҵ����л��ܼ���
��ش�
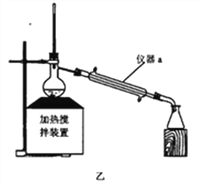
��1����������NaOH���Ȼ������ʵ�װ����_________������a������_________��

��2�����������������������Һ�����������Լ����������������������������ʵ���֮�ȣ��ó�����ʵ�����ݡ�����ʵ��1-3������ʵ�(����)Ϊ_________������ʵ��4-6��n(����):n(��)����ʵ�ѡ��Ϊ3.025��������ʵ��ԭ�ϼ۸�����ܵ������ǣ�_________��
��� | ʱ��/h | n(����)��n(��) | (����) | ���ʣ�%�� | ��� | ʱ��/h | n(����)��n(��) | (����) | ���ʣ�%�� |
1 | 8 | 3.025 | 0��10 | 64.0 | 4 | 10 | 2.935 | 0.20 | 78.4 |
2 | 8 | 3.025 | 0.20 | 72.0 | 5 | 10 | 3.025 | 0.20 | 90.2 |
3 | 8 | 3.025 | 0.30 | 68.5 | 6 | 10 | 3.505 | 0.20 | 91.3 |
��3������I��Է�Ӧ��������ϴ�ӣ�����ϴ��ҺҲ���ˡ����Ǣ���Դֲ�Ʒ����ϴ�ӡ�����ϴ�Ӽ�����ʵķֱ���_________��
A������Iϴ�Ӽ�����ˮ�����Ǣ�ϴ�Ӽ�����ˮ��
B������Iϴ�Ӽ�����Һ�����Ǣ�ϴ�Ӽ�����Һ��
C������Iϴ�Ӽ�������Һ������ˮ�Ҵ������Ǣ�ϴ�Ӽ�����ˮ�Ҵ���
D������Iϴ�Ӽ�������ˮ�Ҵ�������Һ�����Ǣ�ϴ�Ӽ�������ˮ�Ҵ�������Һ��
��4�����������Ȳⶨ�������£�ȡag����������Է�������294����Ʒ�ܽ⣬���뻺����Һ����pHֵ������bmLcmol��L-1��EDTA��Һ��Ȼ�����ָʾ������d mol��L-1�ı�п��Һ�ζ�������EDTA��Һ��Al3+��Zn2+��EDTA��1��1��Ӧ��ʵ�����ı�п��Һ emL��������������Ϊ_________��