��Ŀ����
����A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��Bԭ�ӵ�����������֮����Cԭ�ӵ�������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ����ﳣ���¾�ΪҺ̬����ش��������⣨���ʱ��ʵ�����ʵĻ�ѧʽ��ʾ����
��1��C��F����Ԫ���γ�һ�ֻ�������ӣ���ԭ��������8���ӽṹ����÷��ӵĽṹʽ ����ռ乹��Ϊ ��
��2����ѧ���Ƴ���һ��ֱ������̬������ B2D2���ӣ��Ҹ�ԭ������㶼����8���ӽṹ����B2D2����ʽΪ ��
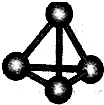
��3��C4���ӽṹ����26ͼ��ʾ����֪����lmol C��C����167kJ����������1mo1C��C�ų�942kJ����.������˵����ȷ���� ��

�� C4����һ�����͵Ļ����� �� C4�е��P4(����)��
�� lmol C4����ת��ΪC2����882kJ���� �� C2��C4��Ϊͬ��������
�� C4��C2��Ϊͬ���칹��
��4����֪A2(g)��C2(g)һ�������·�Ӧ����0.5mol CA3(g)�ų�23kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��5��Ϊ�˳�ȥ��������(A2ED4)��ϡ��Һ�л��е�A2ED3��������������A2D2��������Ӧ�����ӷ���ʽΪ�� ��
��1��![]() (2��) ������ (2��)
(2��) ������ (2��)
��2��![]() (2��)
(2��)
��3�� �ڢ� (2��)
��4��N2(g)+3H2(g)![]() 2NH3(g)�� ��H =-92kJ/mol (3��)
2NH3(g)�� ��H =-92kJ/mol (3��)
��5��H2O2+H2SO3=2H+ ʮSO42��+H2O ��3�֣�

 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






