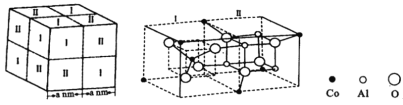
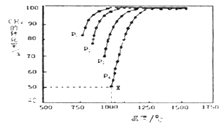
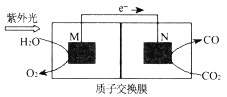
��Ŀ����
����Ŀ��ʵ������Ҫ95 mL l.0 mol/Lϡ���ᣬ����98%��Ũ����(���ܶ�Ϊ1.84 g.mL-l)�����ơ�
(1)ʵ����Ҫ�IJ���������50 mL�ձ�������������ͷ�ιܡ�____��____��
(2)������������Ϊ____����ͷ�ιܵ�����Ϊ________��
(3)���ƹ����У����������ʹ���ƽ��ƫ�͵���(�����)____��
A.��ϡ�͵�����Һת��������ƿ��ϴ���ձ��Ͳ�����2-3�Ρ�
B.���ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�⡣
C.����ƿʹ��ʱδ���
D.�ý�ͷ�ιܼ�ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶����С�
E.δ��ȴ�����¾Ͷ��ݡ�
F.���ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߡ�
���𰸡�100mL����ƿ 10mL��Ͳ �������� ���� BDF
��������
(1)����c=![]() ������98%��Ũ���ᣨ���ܶ�Ϊ1.84g/mL�������ʵ���Ũ�ȣ�������Һϡ��ǰ�����ʵ����ʵ������������ҪŨ����������
������98%��Ũ���ᣨ���ܶ�Ϊ1.84g/mL�������ʵ���Ũ�ȣ�������Һϡ��ǰ�����ʵ����ʵ������������ҪŨ����������
(2)��������һ�����ʵ���Ũ�Ȳ���ѡ����ʵ�������ָ������������;��
(3)�����������������ʵ����ʵ�������Һ�������Ӱ������c=![]() ������������
������������
(1)98%��Ũ����(���ܶ�Ϊ1.84g/mL)�����ʵ���Ũ��=![]() =
=![]() =18.4mol/L������95mL1.0moL1ϡ����Ӧѡ��100mL����ƿ������ҪŨ��������ΪV��������Һϡ��ǰ�����ʵ����ʵ������䣬��V��18.4mol/L=1.0moL1��100mL�����V=5.4mL������һ�����ʵ���Ũ�Ȳ��裺���㡢��ȡ��ϡ�ͣ���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������10mL��Ͳ������������ͷ�ιܡ��ձ���100mL����ƿ��
=18.4mol/L������95mL1.0moL1ϡ����Ӧѡ��100mL����ƿ������ҪŨ��������ΪV��������Һϡ��ǰ�����ʵ����ʵ������䣬��V��18.4mol/L=1.0moL1��100mL�����V=5.4mL������һ�����ʵ���Ũ�Ȳ��裺���㡢��ȡ��ϡ�ͣ���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������10mL��Ͳ������������ͷ�ιܡ��ձ���100mL����ƿ��
(2) ϡ��ʱ�ò��������裬��Һʹ�ò���������������ʱ���ý�ͷ�ιܶ��ݣ������ǵμ�����Һ�壻��Ϊ���裬���������ݣ�
(3) A����ϡ�͵�����Һת��������ƿ��ϴ���ձ��Ͳ�����2-3�β�����ȷ��û������A���������⣻
B�����ձ��ڵ�ϡ����������ƿ��ת��ʱ�����������ʹ����ϡ���ὦ��ƿ�⣬�������ʵ����ʵ���ƫС����Һ��Ũ��ƫС����B�������⣻
C������ƿʹ��ʱδ����,���滹Ҫ���ݣ�������Һ�岻Ӱ��ʵ������C���������⣻
D���ý�ͷ�ιܼ�ˮʱ�����ӹ۲���Һ��Һ��������ƿ�̶����У�������Һ�����ƫ����Һ��Ũ��ƫС����D�������⣻
E��δ��ȴ�����¾Ͷ��ݣ���ȴ��Һ����ڿ̶��ߣ���Һ�����ƫС����Һ��Ũ��ƫ��C���������⣻
F�����ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫС����F�������⣻
�ʴ�ѡ��BDF��

 ��У����ϵ�д�
��У����ϵ�д�