��Ŀ����
����Ŀ��Ϊ�ⶨ���ȷ�Ӧʵ���������������ijɷ֣�ijѧ����ȤС�����ʵ��������ͼ��ʾ��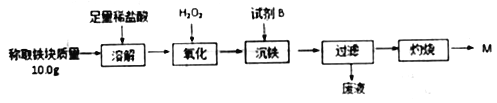
�����������↑ʼ��������ȫ������pH���±���ʾ��
Fe2+ | Fe3+ | Al3+ | Mg2+ | |
��ʼ����ʱ��pH | 7.5 | 2.8 | 4.2 | 9.6 |
������ȫʱ��pH | 9.0 | 4.0 | 5 | 11 |
(1)ȡ�ܽ�����õ�����Һ�������еμ�KSCN��Һ��������Һ��Ѫ��ɫ���������������ԭ���˿��ܻ���û��Ӧ����������⣬����һ��ԭ����_______________��
(2)��������Ӧ������ҺpH�ķ�Χ��Ϊ________���Լ�B ѡ��______��(�����)
a.ϡ���� b.������ c.MgCO3���� d.��ˮ
(3)��֪������Fe(OH)3��Ksp=1.1��10-36��������ȫ����ʱ��Һ��c(Fe3+)=_____mol/L��
(4)�������̵õ��Ĺ���M����_____(��ܡ����ܡ�)����������н�������������ԭ����________________��
(5)����С����õζ��ķ�ʽ�ⶨ�����顱�����ĺ���(��Ԫ��ֻ����Fe��Fe2O3)���ⶨ��ʽ���£�
I.ȡ10g�����顱���飬������������������Һ��ַ�Ӧ�����ˡ�ϴ�ӡ�����õ�8.88g���塣������������������ܽ⣬���200mL��ҺA��
II.ȡ��10mL ��ҺA ����ƿ���ȼ���������SnCl2��Һ����Fe3+ȫ��ת��ΪFe2+����ȥ������Sn2+��
III.����һ��������������ᣬ���μ�ָʾ����������ƿ�еμ�0.1000mol/LK2Cr2O7��Һ��ǡ����ȫ��Ӧʱ��������12.50mL K2Cr2O7��Һ��������Ӧ:Cr2O72-+6Fe2++14H+==2Cr3++6Fe3++7H2O
�ٲ���I�м�����������������Һ������Ϊ________________________��
���������Ʒ��Fe���ʵ���������__________(д���������)��
���𰸡� ���ڵ������������������� 4~4.2 d 1.1��10��6 ���� �����л����ܺ��������������� ʹ������������������ȫ�ܽ� 72.8%
��������(1)ȡ�ܽ�����õ�����Һ�������еμ�KSCN��Һ��������Һ��Ѫ��ɫ��˵����Һ�д���Fe3+����������û��Ӧ�����������Ҳ�п��������ڵ����������������������ʴ�Ϊ�����ڵ���������������������
(2)����������⣬����Һ�е���������ȫ���������������ӣ������������↑ʼ��������ȫ������pH�����ݿ�֪����������ʱ���ܽ������ӳ�����Ӧ������ҺpH�ķ�ΧΪ4~4.2������ϡ���ᣬ��Һ��������ǿ���ﲻ��������Ŀ�ģ�����������������Ϊ�������ɫ��δM������ƫ��Ҳ������MgCO3���壬��ΪMgCO3�������ʱ���������ù���M������ƫ��Ӧѡ�ð�ˮ����ѡd�����ʴ�Ϊ��4~4.2��d��
(3)����Fe(OH)3��Ksp=c(Fe3+)c3(OH-)=1.1��10-36����pH=4����c(OH-)=10-10mol/L������Ksp=c(Fe3+)c3(OH-)=1.1��10-36�������c(Fe3+)=1.1��10-6mol/L���ʴ�Ϊ��1.1��10-6��
(4)�������̵õ��Ĺ���M�п��ܺ��������������ʣ���˲��ܸ���M����������������н��������������ʴ�Ϊ�����ܣ������л����ܺ��������������ʣ�
(5)�ٲ���I�м�����������������Һ������ʹ�����е�������������������ȫ�ܽ⣬�ʴ�Ϊ��ʹ������������������ȫ�ܽ⣻
�ڵζ������ĵ�K2Cr2O7�����ʵ���=0.01250L��0.1000mol/L=0.00125mol������Cr2O72-+6Fe2++14H+==2Cr3++6Fe3++7H2O��������Ԫ���غ㣬10mL��ҺA�к��е�Fe3+�����ʵ���=0.00125mol��6=0.0075mol����200mL��ҺA�к��е�Fe3+�����ʵ���=0.0075mol��20=0.15mol����Fe��Fe2O3�����ʵ����ֱ�Ϊx��y����x+2y=0.15mol��56g/mol��x+160 g/mol��y=8.88g�����x=0.13mol��y=0.01mol����Ʒ��Fe���ʵ���������=![]() ��100%= 72.8%���ʴ�Ϊ��72.8%��
��100%= 72.8%���ʴ�Ϊ��72.8%��
