��Ŀ����
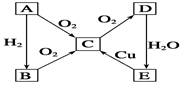
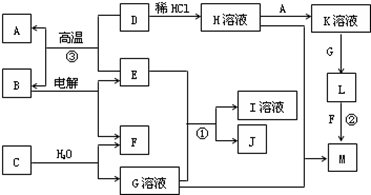
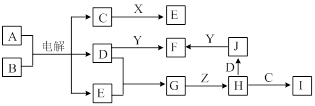
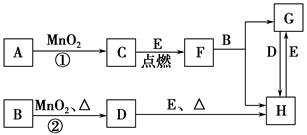
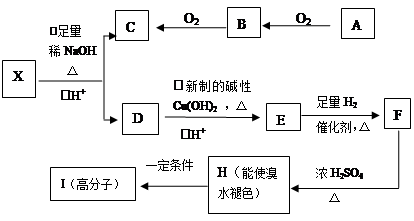
A��YΪ�����������ʣ�XΪ�����ǽ������ʡ�������X��G��H��IΪ���壬CΪҺ�塣B��������Ԫ����ɵ��Σ�����ʱ�����ֽ������������壬��ȴ���ֿɻ��ϵõ�B���й�����֮���ת����ϵ����ͼ(���ַ�Ӧ��������������ȥ)��
����д���пհף�
(1)B�ĵ���ʽΪ____________________________��
(2)����A��ʯī���缫��B��Ũ��Һ���������Һ������ԭ��ء���������ӦʽΪ____________________��
(3)��Ӧ�Ļ�ѧ����ʽΪ__________________________����Ӧ����ұ��ҵ������________________(�������ұ������)��
(4)��D�Ľᾧˮ�����Ʊ�D����ˮ����IJ���Ϊ_____________________________��
(5)��Ӧ�ڵĻ�ѧ����ʽΪ________________________________________________��
��Ӧ�۵����ӷ���ʽΪ___________________________________________________��
(1) 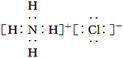
(2)2NH4+��2e��=2NH3����H2��
(3)3Fe��4H2O(g) Fe3O4��4H2���Ȼ�ԭ�������Ȼ�ԭ��
Fe3O4��4H2���Ȼ�ԭ�������Ȼ�ԭ��
(4)��D�Ľᾧˮ������HCl�����м���
(5)4NH3��5O2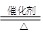 4NO��6H2O��Al3����3NH3��H2O=Al(OH)3����3NH4+ [��Al3����3NH3��3H2O=Al(OH)3����3NH4+]
4NO��6H2O��Al3����3NH3��H2O=Al(OH)3����3NH4+ [��Al3����3NH3��3H2O=Al(OH)3����3NH4+]
����