��Ŀ����
17������˵����ȷ���ǣ��������ٱ�״���£�2.24LNO2 ��ˮ��Ӧ����NO3-����ĿΪ0.1NA
��������Ϊ92��������Ϊ143���ˣ�U��ԭ�ӣ�92235U
��������Һ��ͨ��������CO2��CO2+H2O+2C6H5O-��2C6H5OH+2CO32-
������ˮ�������c��OH-��=10-13 mol•L-1����Һ�У�Na+��Ba2+��Cl-��I-�ɴ�������
��0.1mol�屽�к���˫������ĿΪ0.3NA
��1��3-�������飮
| A�� | �٢ڢ� | B�� | �ڢܢ� | C�� | �ڢ� | D�� | �ۢݢ� |
���� �ٱ�״����2.24LNO2�����ʵ���Ϊ0.1mol�����ڶ���������ˮ��Ӧ����NO�����ᣬ����������������ʵ���С��0.1mol����
��������=������+��������Ԫ�ط��ŵ����Ͻ�Ϊ�����������½�Ϊ��������
�۱��ӵ����Դ���̼��������ӣ����߷�Ӧ���ɱ��Ӻ�̼��������ӣ�
������ˮ�������c��OH-��=10-13 mol•L-1����Һ�д��ڴ��������ӻ����������ӣ���������֮�䲻��Ӧ�������������Ӻ����������ӷ�Ӧ��
���屽�в�����̼̼˫����
�������������У������ܳ���1����˵��ѡȡ�����������̼����
��� �⣺�ٱ�״���£�2.24LNO2�����ʵ���Ϊ0.1mol��0.1mol����������ˮ��Ӧ���������NO����Ӧ������������ӵ����ʵ���С��0.1mol��NO3-����ĿС��0.1NA���ʢٴ���
��������Ϊ92��������Ϊ143���ˣ�U��ԭ�ӵ�������Ϊ235���ú��ؿ��Ա�ʾΪ��92235U���ʢ���ȷ��
��������Һ��ͨ��������CO2�����ڱ��ӵ����Դ���̼��������ӣ����߷�Ӧ���ɱ��Ӻ�̼��������ӣ���ȷ�����ӷ���ʽΪ��CO2+H2O+C6H5O-��C6H5OH+HCO3-���ʢ۴���
������ˮ�������c��OH-��=10-13 mol•L-1����ҺΪ���Ի������Һ����Һ�д��ڴ��������ӻ����������ӣ�Na+��Ba2+��Cl-��I-֮�䲻��Ӧ�������������Ӻ����������ӷ�Ӧ������Һ���ܹ��������棬�ʢ���ȷ��
�ݱ����в�����̼̼˫������0.1mol�屽������˫�����ʢݴ���
��1��3-�������飺�������д���1-����˵��ѡȡ������������ȷ����Ӧ��Ϊ��2-�����飬�ʢ���
���ݷ�����֪����ȷ��Ϊ���ڢܣ�
��ѡC��
���� ���⿼���˰���٤�����������ӷ���ʽ�����ӹ�����жϡ��л���������֪ʶ����Ŀ�Ѷ��еȣ�����֪ʶ��϶࣬��ֿ���ѧ�����Ӧ�û���֪ʶ��������ע���������ӷ���ʽ����дԭ�����ӹ���������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | HCl��H2SO4�������� | B�� | K2CO3��K2O�������� | ||
| C�� | NaOH��Na2CO3�����ڼ� | D�� | H2O��O2������������ |
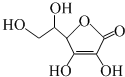
| A�� | ά����C�����Ĺ��������ǻ����Ȼ���̼̼˫�� | |
| B�� | ά����C�ܺ���ˮ�������ظ������Һ��Ӧ | |
| C�� | ά����C�ķ���ʽΪC6H6O6 | |
| D�� | ά����C�ܷ����ӳɷ�Ӧ��������Ӧ�����ܷ���ȡ����Ӧ |
��1����PM2.5����������ˮ�����Ƴɴ�����������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��NOx ����β������Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�
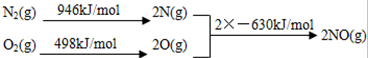
��N2��O2��Ӧ����NO���Ȼ�ѧ��Ӧ����ʽΪN2��g��+O2��g��=2NO��g����H=+184kJ•mol-1
��3����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ�����Ƶ������������������£�
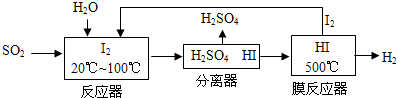
�������ӷ���ʽ��ʾ��Ӧ���з����ķ�Ӧ��SO2+I2+2H2O=SO42-+2I-+4H+
�ڽ����ɵ������������ֱ�ͨ����������Ե缫��KOH��Һ��Ϊ�������Һ�������ĵ缫��ӦʽH2-2e-+2OH-=2H2O
��4��Ϊ�˸��ƻ������й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��50%��
����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���C������ţ���
A�����ˮ���⣺2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2��B������ʹˮ�ֽ����⣺2H2O��g��$\frac{\underline{\;����\;}}{\;}$2H2+O2
C��̫������ֽ�ˮ���⣺2H2O$\frac{\underline{\;\;\;TiO_{2}\;\;\;}}{̫����}$2H2��+O2��
D����Ȼ�����⣺CH4+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO+3H2
��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H=-49.0kJ•mol-1�����CO2��CH3OH��g��Ũ����ʱ��仯��ͼ��ʾ����3min��9min��v��H2��=0.125mol•L-1•min-1��
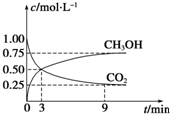
����˵��������Ӧ�ﵽƽ��״̬����D�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1
����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3mol H2��ͬʱ����1mol H2O
D��CO2����������ڻ�������б��ֲ���
�ܹ�ҵ�ϣ�CH3OHҲ����CO��H2�ϳɣ��ο��ϳɷ�ӦCO��g��+2H2��g��?CH3OH��g����ƽ�ⳣ��������˵����ȷ����AC��
| �¶�/�� | 0 | 100 | 200 | 300 | 400 |
| ƽ�ⳣ�� | 667 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
B���÷�Ӧ�ڵ����²����Է����У������¿��Է�����
C����T��ʱ��1L�ܱ������У�Ͷ��0.1mol CO��0.2mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ��5MPa����250�棬����Ϊ�������£�ԭ����ת������ߣ�
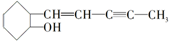 ���ҹ���ѧ������ºϳɵ�һ��ҩ����й��ڸ��л����˵��������ǣ�������
���ҹ���ѧ������ºϳɵ�һ��ҩ����й��ڸ��л����˵��������ǣ�������| A�� | ���л���ķ���ʽΪC11H16O | |
| B�� | ���л������ʹ��ˮ��ɫ | |
| C�� | ���л���������ˮ | |
| D�� | ���գ����л����������������ȡ����Ӧ |
| A�� | Y��Z��RԪ�ؼ����ӵİ뾶�������� | |
| B�� | ����Y��Z��R����Ԫ�صĻ��������ֻ��2�� | |
| C�� | Ԫ��W��R����������Ӧˮ��������Ժ���ǿ | |
| D�� | Y��Z�γɵ����ֻ������еĻ�ѧ�����ͺ��������Ӹ����Ⱦ���ͬ |
| A�� | 8 | B�� | 16 | C�� | 10 | D�� | 12 |
 ��ѧ�е�ijЩԪ����������ܲ��ɷ֣���ش��������⣺
��ѧ�е�ijЩԪ����������ܲ��ɷ֣���ش��������⣺