ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΖζΦΑΤδΜ·ΚœΈο”ΟΆΨ °Ζ÷ΙψΖΚΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΖζΜ·ΈοOF2ΓΔNF3ΓΔSiF4ΓΔPF5ΓΔSF6÷–Θ§÷––Ρ‘≠Ή”≤…»Γsp3‘”Μ·ΒΡ «__ΓΘ
Θ®2Θ©[H2F]+[SbF6]-(ΖζΧύΥα) «“Μ÷÷≥§«ΩΥαΓΘΧύΒΡΦέΒγΉ”≈≈≤Φ ΫΈΣ__ΓΘ―τάκΉ”[H2F]+ΒΡΩ’ΦδΙΙ–ΆΈΣ__Θ§–¥≥ω[H2F]+ΒΡΒ»ΒγΉ”Χε__Θ®Ζ÷Ή”ΚΆάκΉ”ΗςΨΌ“ΜάΐΘ©ΓΘ
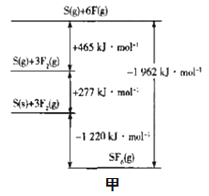
Θ®3Θ©SF6±ΜΙψΖΚ”Ο”ΎΗΏ―ΙΒγΤς…η±ΗΒΡΨχ‘ΒΫι÷ ΓΘΗυΨί__άμ¬έΘ§Ω…≈–Εœ≥ωΤδΩ’ΦδΙΙ–ΆΈΣ’ΐΑΥΟφΧεΓΘSF6ΒΡΦϋΡήΩ…Ά®ΙΐάύΥΤBorn-Haber―≠ΜΖΡήΝΩΙΙΫ®ΡήΝΩΆΦΘ®ΆΦΦΉΘ©ΦΤΥψΦϋΡήΘ§‘ρS-FΒΡΦϋΡήΈΣ___kJmol-1ΓΘ
Θ®4Θ©ΙΛ“Β…œΒγΫβAl2O3÷Τ»ΓΒΞ÷ ¬ΝΘ§≥Θάϊ”Ο±υΨß ·Na3[AlF6]ΫΒΒΆAl2O3ΒΡ»έΒψΓΘ±υΨß ·ΒΡ…ζ≤ζ‘≠άμΈΣ2Al(OH)3+12HF+3Na2CO3=2Na3[AlF6]+3CO2Γϋ+9H2OΓΘ
ΔΌ≤βΕ®ΤχΧ§HFΒΡΡΠΕϊ÷ ΝΩ ±Θ§ΆυΆυΒΟ≤ΜΒΫ20gmol-1ΒΡ ΐΨίΘ§‘≠“ρ «__ΓΘ
ΔΎ±υΨß ·ΒΡΨßΧε≤ΜΒΦΒγΘ§ΒΪ»έ»Ύ ±ΡήΒΦΒγΘ§‘ρ‘Ύ±υΨß ·ΨßΧε÷–¥φ‘Ύ__(Χν–ρΚ≈)ΓΘ
a.άκΉ”Φϋ b.ΦΪ–‘Φϋ c.≈δΈΜΦϋ d.ΖΕΒ¬ΜΣΝΠ
ΔέΖ¥”ΠΈο÷–‘ΣΥΊΘ®«β≥ΐΆβΘ©ΒΡΒΎ“ΜΒγάκΡή¥”¥σΒΫ–ΓΒΡΥ≥–ρΈΣ__(”Ο‘ΣΥΊΖϊΚ≈±μ Ψ)ΓΘ
ΔήΙΛ“Β…œ≤Μ”ΟΒγΫβ»έΒψΗϋΒΆΒΡAlCl3÷Τ»Γ¬ΝΒΡ‘≠“ρΈΣ__ΓΘ
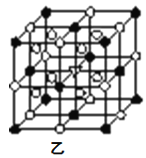
Δί±υΨß ·”…ΝΫ÷÷ΈΔΝΘΙΙ≥…Θ§±υΨß ·ΒΡΨßΑϊΫαΙΙ»γΆΦ““Υυ ΨΘ§ΓώΈΜ”Ύ¥σΝΔΖΫΧεΒΡΕΞΒψΚΆΟφ–ΡΘ§ΓπΈΜ”Ύ¥σΝΔΖΫΧεΒΡ12ΧθάβΒΡ÷–ΒψΚΆ8Ηω–ΓΝΔΖΫΧεΒΡΧε–ΡΘ§Ρ«Ο¥¥σΝΔΖΫΧεΒΡΧε–Ρ¥ΠΥυ¥ζ±μΒΡΈΔΝΘ «__(ΧνΨΏΧεΒΡΈΔΝΘΖϊΚ≈)ΓΘ
ΓΨ¥πΑΗΓΩOF2ΓΔNF3ΓΔSiF4 5s25p3 V–Ά H2OΜρH2SΓΔNH2- Φέ≤ψΒγΉ”ΜΞ≥β 327 ≤ΩΖ÷ΤχΧ§HFΖ÷Ή”Φδ“‘«βΦϋΫαΚœΝΥ abc FΘΨOΘΨCΘΨAlΘΨNa AlCl3ΈΣΖ÷Ή”ΨßΧεΘ§»έ»Ύ≤ΜΒΦΒγΘ§ΈόΖ®ΒγΫβ Na+
ΓΨΫβΈωΓΩ
(1)OF2÷–O–Έ≥…2ΗωΠΡΦϋΘ§Ι¬ΒγΉ”Ε‘=![]() =2Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ2+2=4Θ§Υυ“‘OF2ΈΣsp3‘”Μ·Θ§Ι ΖϊΚœΧβ“βΘΜNF3÷–N–Έ≥…3ΗωΠΡΦϋΘ§Ι¬ΒγΉ”Ε‘=
=2Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ2+2=4Θ§Υυ“‘OF2ΈΣsp3‘”Μ·Θ§Ι ΖϊΚœΧβ“βΘΜNF3÷–N–Έ≥…3ΗωΠΡΦϋΘ§Ι¬ΒγΉ”Ε‘=![]() =1Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ1+3=4Θ§Υυ“‘ΈΣsp3‘”Μ·Θ§Ι ΖϊΚœΧβ“βΘΜ SiF4÷–Si–Έ≥…4ΗωΠΡΦϋΘ§ΟΜ”–Ι¬ΒγΉ”Ε‘Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ0+4=4Θ§Υυ“‘ΈΣsp3‘”Μ·Θ§Ι ΖϊΚœΧβ“βΘΜPF5÷–P–Έ≥…5ΗωΠΡΦϋΘ§ΟΜ”–Ι¬ΒγΉ”Ε‘Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ0+5=5Θ§Υυ“‘ΈΣsp3d‘”Μ·Θ§Ι ≤ΜΖϊΚœΧβ“βΘΜSF6÷–S–Έ≥…6ΗωΠΡΦϋΘ§Ι¬ΒγΉ”Ε‘=
=1Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ1+3=4Θ§Υυ“‘ΈΣsp3‘”Μ·Θ§Ι ΖϊΚœΧβ“βΘΜ SiF4÷–Si–Έ≥…4ΗωΠΡΦϋΘ§ΟΜ”–Ι¬ΒγΉ”Ε‘Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ0+4=4Θ§Υυ“‘ΈΣsp3‘”Μ·Θ§Ι ΖϊΚœΧβ“βΘΜPF5÷–P–Έ≥…5ΗωΠΡΦϋΘ§ΟΜ”–Ι¬ΒγΉ”Ε‘Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ0+5=5Θ§Υυ“‘ΈΣsp3d‘”Μ·Θ§Ι ≤ΜΖϊΚœΧβ“βΘΜSF6÷–S–Έ≥…6ΗωΠΡΦϋΘ§Ι¬ΒγΉ”Ε‘=![]() =0Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ0+6=6ΈΣsp3d2‘”Μ·Θ§Ι ≤ΜΖϊΚœΧβ“βΘΜΙ ¥πΑΗΈΣΘΚOF2ΓΔNF3ΓΔSiF4ΘΜ
=0Θ§ΉήΙ≤ΒγΉ”Ε‘ ΐΈΣ0+6=6ΈΣsp3d2‘”Μ·Θ§Ι ≤ΜΖϊΚœΧβ“βΘΜΙ ¥πΑΗΈΣΘΚOF2ΓΔNF3ΓΔSiF4ΘΜ
(2)ΧύΈΣΒΎΈε÷ήΤΎΒΎV÷ςΉε‘ΣΥΊΘ§ΤδΦέΒγΉ”≈≈≤Φ ΫΈΣ5s25p3ΓΘ[H2F]+”κH2OΓΔNH2-ΜΞΈΣΒ»ΒγΉ”ΧεΘ§ΫαΙΙœύΥΤΘ§ΤδΩ’ΦδΙΙ–ΆΨυΈΣΘΚV–ΆΘ§Ι ¥πΑΗΘΚ5s25p3ΘΜV–ΆΘΜH2OΓΔNH2-ΘΜ
(3)SF6±ΜΙψΖΚ”Ο”ΎΗΏ―ΙΒγΤς…η±ΗΒΡΨχ‘ΒΫι÷ ΓΘΗυΨίΦέ≤ψΒγΉ”ΜΞ≥βάμ¬έΘ§Ω…≈–Εœ≥ωΤδΩ’ΦδΙΙ–ΆΈΣ’ΐΑΥΟφΧεΓΘΗυΨίBorn-Haber―≠ΜΖΡήΝΩΙΙΫ®ΡήΝΩΆΦΩ…÷ΣΘ§S(s)+3F2(g)![]() SF6ΒΡS-FΒΡΦϋΡή=
SF6ΒΡS-FΒΡΦϋΡή=![]() =327kJΓΛmol-1ΓΘΙ ¥πΑΗΈΣΘΚΦέ≤ψΒγΉ”ΜΞ≥βΘΜ327ΘΜ
=327kJΓΛmol-1ΓΘΙ ¥πΑΗΈΣΘΚΦέ≤ψΒγΉ”ΜΞ≥βΘΜ327ΘΜ
(4)ΔΌ“ρΈΣHFΖ÷Ή”Φδ¥φ‘Ύ«βΦϋΘ§Υυ“‘‘Ύ≤βΕ®ΤχΧ§HFΒΡΡΠΕϊ÷ ΝΩ ±Θ§”–≤ΩΖ÷HFΖ÷Ή”Ά®Ιΐ«βΦϋΕχΫαΚœΝΥΘ§ΆυΆυΒΟ≤ΜΒΫ20gmol-1ΒΡ ΐΨίΘ§Ι ¥πΑΗΘΚ”–≤ΩΖ÷HFΖ÷Ή”Ά®Ιΐ«βΦϋΕχΫαΚœΝΥΘΜ
ΔΎ±υΨß ·(Na3AlF6)ΨßΧε≤ΜΒΦΒγΘ§ΒΪ»έ»Ύ ±ΡήΒΦΒγΘ§ΥΒΟς τ”ΎάκΉ”Μ·ΚœΈοΘ§Κ§”–άκΉ”ΦϋΘ§”…Na+ΓΔ[AlF6]3-ΙΙ≥…Θ§[AlF6]3-÷–Κ§”–≈δΈΜΦϋΘ§“≤ τ”ΎΦΪ–‘ΦϋΘ§Ι ―ΓΘΚabcΘΜ
Δέ±υΨß ·ΒΡ…ζ≤ζ‘≠άμΈΣ2Al(OH)3+12HF+3Na2CO3=2Na3[AlF6]+3CO2Γϋ+9H2OΘ§Ζ¥”ΠΈο÷–‘ΣΥΊ(«β≥ΐΆβ)ΜΙ”–AlΓΔOΓΔFΓΔNaΓΔC‘ΣΥΊΘ§ΗυΨίΖ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΤδΒγΗΚ–‘‘Ϋ«ΩΘ§ΒγΝΠΡή‘Ϋ¥σΘ§Υυ“‘ΥϊΟ«ΒΡΒΎ“ΜΒγάκΡή¥”¥σΒΫ–ΓΒΡΥ≥–ρΈΣFΘΨOΘΨCΘΨAlΘΨNaΘΜ
Δή“ρΈΣAlCl3ΈΣΙ≤Ϋ®Μ·ΚœΈοΘ§ τ”ΎΖ÷Ή”ΨßΧεΘ§‘Ύ»έ»ΎΉ¥Χ§œ¬Θ§≤ΜΡήΒγάκ≥ωAl3+Θ§Υυ“‘ΙΛ“Β…œ≤Μ”ΟΒγΫβ»έΒψΗϋΒΆΒΡAlCl3÷Τ»Γ¬ΝΘ§Ι ¥πΑΗΘΚ“ρΈΣAlCl3 τ”ΎΖ÷Ή”ΨßΧεΘ§‘Ύ»έ»ΎΉ¥Χ§œ¬Θ§≤ΜΡήΒγάκ≥ωAl3+ΘΜ
ΔίΓώΒΡΗω ΐ=8![]() +6
+6![]() =4Θ§ΓπΗω ΐ=12
=4Θ§ΓπΗω ΐ=12![]() +8=11Θ§“Σ ΙΝΫ÷÷άκΉ”ΒΡΗω ΐ÷°±»ΈΣ1:3Θ§‘ρ¥σΝΔΖΫΧεΒΡΧε–Ρ¥ΠΥυ¥ζ±μΒΡΈΔΝΘ «Na+Θ§Ι ¥πΑΗΈΣΘΚNa+ΘΜ
+8=11Θ§“Σ ΙΝΫ÷÷άκΉ”ΒΡΗω ΐ÷°±»ΈΣ1:3Θ§‘ρ¥σΝΔΖΫΧεΒΡΧε–Ρ¥ΠΥυ¥ζ±μΒΡΈΔΝΘ «Na+Θ§Ι ¥πΑΗΈΣΘΚNa+ΘΜ

 –¬±ύ–Γ―ßΒΞ‘ΣΉ‘≤βΧβœΒΝ–¥πΑΗ
–¬±ύ–Γ―ßΒΞ‘ΣΉ‘≤βΧβœΒΝ–¥πΑΗ Ή÷¥ ΨδΕΈΤΣœΒΝ–¥πΑΗ
Ή÷¥ ΨδΕΈΤΣœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΆ®Ιΐ―ßœΑΘ§Ά§―ßΟ«Ε‘ΚξΙέ±φ Ε”κΈΔΙέΧΫΈωΘ§±δΜ·ΙέΡν”κΤΫΚβΥΦœκΒ»―ßΩΤΥΊ―χ”–ΝΥΫχ“Μ≤ΫΒΡ»œ ΕΚΆάμΫβΓΘ«κΗυΨίΥυ―ß÷Σ ΕΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©«Π–νΒγ≥Ί «Βδ–ΆΒΡΩ…≥δΒγ–ΆΒγ≥ΊΘ§Βγ≥ΊΉήΖ¥”Π ΫΈΣΘΚPbΘΪPbO2ΘΪ4HΘΪΘΪ2SO42-![]() 2PbSO4 ΘΪ2H2OΓΘΖ≈Βγ ±Θ§ΗΚΦΪΒΡΒγΦΪΖ¥”Π Ϋ «_______ΘΜ≥δΒγ ±Θ§Β±ΆβΒγ¬ΖΆ®Ιΐ 0.2 mol ΒγΉ” ±Θ§άμ¬έ…œ’ΐΦΪΑεΒΡ÷ ΝΩΦθ…Ό_________gΓΘ
2PbSO4 ΘΪ2H2OΓΘΖ≈Βγ ±Θ§ΗΚΦΪΒΡΒγΦΪΖ¥”Π Ϋ «_______ΘΜ≥δΒγ ±Θ§Β±ΆβΒγ¬ΖΆ®Ιΐ 0.2 mol ΒγΉ” ±Θ§άμ¬έ…œ’ΐΦΪΑεΒΡ÷ ΝΩΦθ…Ό_________gΓΘ
Θ®2Θ©“―÷ΣΘΚ≥ΘΈ¬œ¬Θ§Ksp[MgΘ®OHΘ©2]ΘΫ1.8ΓΝ10-11Θ§Ksp[FeΘ®OHΘ©3]ΘΫ4ΓΝ10-38ΓΘ
ΔΌ≥ΘΈ¬œ¬Θ§Ρ≥Υα–‘ MgCl2 »ή“Κ÷–Κ§”–…ΌΝΩΒΡ FeCl3 Θ§ΈΣΝΥΒΟΒΫ¥ΩΨΜΒΡ MgCl2ΓΛ2H2O ΨßΧεΘ§”ΠΦ”»κ_________ΧνΜ·―ß ΫΘ©Θ§ΒςΫΎ»ή“ΚΒΡ pHΘΫ4Θ§ Ι»ή“Κ÷–ΒΡ Fe3+ΉΣΜ·ΈΣ FeΘ®OHΘ©3≥ΝΒμΘ§¥Υ ±»ή“Κ÷–ΒΡ cΘ®Fe3+Θ©ΘΫ_________molΓΛL-1ΓΘ
ΔΎ≥ΘΈ¬œ¬Θ§»τΫΪ 0.01 molΓΛL-1 MgCl2 »ή“Κ”κ÷Ν…Ό________molΓΛL-1 NaOH »ή“ΚΒ»ΧεΜΐΜλΚœ ±”–≥ΝΒμ…ζ≥…ΓΘ
Θ®3Θ©25Γφ ±Θ§ΦΗ÷÷άκΉ”ΩΣ Φ≥ΝΒμ ±ΒΡ pH »γœ¬±μΘΚ
άκΉ” | Fe2+ | Cu2+ | Mg2+ |
pH | 7.6 | 5.2 | 10.4 |
Β±œρΚ§œύΆ§≈®Ε» Cu2+ΓΔMg2+ΓΔFe2+ΒΡ»ή“Κ÷–ΒΈΦ” NaOH »ή“Κ ±Θ§__________œ»≥ΝΒμΘ®ΧνάκΉ”ΖϊΚ≈Θ©Θ§“Σ Ι 0.3molΓΛL-1 ΝρΥαΆ≠»ή“Κ÷–Cu2+≥ΝΒμΫœΈΣΆξ»ΪΘ®Β± Cu2+≈®Ε»ΫΒ÷Ν 10-5 molΓΛL-1 ±Θ©Θ§‘ρ”Πœρ»ή“ΚάοΦ”»κ«β―θΜ·ΡΤ»ή“Κ Ι»ή“Κ pH ΈΣ________Θ®KspCuΘ®OHΘ©2=1ΓΝ10-20Θ©
ΓΨΧβΡΩΓΩT1Έ¬Ε» ±‘Ύ»ίΜΐΈΣ2LΒΡΚψ»ίΟή±’»ίΤς÷–÷Μ≥δ»κ1.00molNO2ΤχΧεΖΔ…ζΖ¥”ΠΘΚ2NO(g)+O2(g)![]() 2NO2(g) H<0ΓΘ Β―ι≤βΒΟΘΚv’ΐ=k’ΐc2(NO)ΓΛc(O2)Θ§vΡφ=kΡφc2(NO2)Θ§k’ΐΓΔkΡφΈΣΥΌ¬ ≥Θ ΐ÷Μ ήΈ¬Ε»”ΑœλΓΘ≤ΜΆ§ ±ΩΧ≤βΒΟ»ίΤς÷–n(NO2)»γœ¬±μΘΚ
2NO2(g) H<0ΓΘ Β―ι≤βΒΟΘΚv’ΐ=k’ΐc2(NO)ΓΛc(O2)Θ§vΡφ=kΡφc2(NO2)Θ§k’ΐΓΔkΡφΈΣΥΌ¬ ≥Θ ΐ÷Μ ήΈ¬Ε»”ΑœλΓΘ≤ΜΆ§ ±ΩΧ≤βΒΟ»ίΤς÷–n(NO2)»γœ¬±μΘΚ
±Φδ/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO2)/mol | 1.00 | 0.80 | 0.65 | 0.55 | 0.50 | 0.50 |
Θ®1Θ©¥”0ΓΪ2sΗΟΖ¥”ΠΒΡΤΫΨυΥΌ¬ v(NO2)=__ΓΘ
Θ®2Θ©T1Έ¬Ε» ±Μ·―ßΤΫΚβ≥Θ ΐK=__LΓΛmol-1ΓΘ
Θ®3Θ©Μ·―ßΤΫΚβ≥Θ ΐK”κΥΌ¬ ≥Θ ΐk’ΐΓΔkΡφΒΡ ΐ―ßΙΊœΒ «K=__ΓΘ»τΫΪ»ίΤςΒΡΈ¬Ε»ΗΡ±δΈΣT2 ±Τδk’ΐ=kΡφΘ§‘ρT2__T1(ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΜρΓΑ=Γ±)ΓΘ