��Ŀ����
��2molSO2��1 mol O2�����������ɱ䣬ѹǿ�㶨���ܱ������У���һ���¶��·������·�Ӧ��2SO2(g)+O2(g)(1)��Ӧ���е�t1ʱ��SO2���������___________;
(2)����t1ʱ����һ���������(Ar)��SO2�����ʵ�����___________(�������С�����䡱)��
(3)����t1ʱ���£����´ﵽƽ��״̬����ƽ������������������ʵ���___________2.1 mol(�������������=��)������˵��ԭ��____________________________________________��
(4)�������������䣬��t1ʱ�ټ���0.2 molSO2��0.1 mol O2��1.8 mol SO3����ͼ��������t0![]() t1
t1![]() t2�����ʱ����SO2�����ʵ����仯���ߡ�
t2�����ʱ����SO2�����ʵ����仯���ߡ�
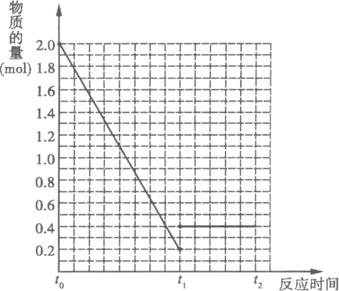
(1)9.5��
(2)����
(3)�� �����¶ȣ���Ӧ�����ȷ�����У���ƽ�����淴Ӧ�����ƶ�������������ʵ�������
(4)����ͼ��ʾ��ע��t0��t1ֻҪ���������(0,2)��ƽ���������(t1,0.2)����ʾ��ͼ������;t1��t2��ƽ����ʱ�����ֱ�ߣ���������(0.4��t1)��


��ϰ��ϵ�д�
�����Ŀ
 ��2molSO2��1molO2�����������ɱ䣬ѹǿ�㶨���ܱ������У���һ���¶��·������·�Ӧ��2SO2��g��+O2��g��
��2molSO2��1molO2�����������ɱ䣬ѹǿ�㶨���ܱ������У���һ���¶��·������·�Ӧ��2SO2��g��+O2��g��