��Ŀ����
8��ij��ѧ����С��������Ϸ��֣�ʵ��������ϩ�ķ�Ӧԭ��ΪCH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH�TCH2��+H2O���Ƶõ���ϩ����������CO2 ��SO2 ��ˮ����������������װ�����һ��ʵ�飬����֤��ϩ��������������ijɷ֣�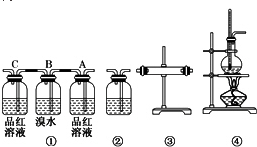
��ش��������⣺
��1��װ�õ�����˳���������������ҵ�����Ϊ�ܢۢ٢ڣ�
��2��װ�â��У�Aƿ�е�������Ʒ����ɫ������Ϊ���������SO2��Bƿ�е������dz�ɫ��ȥ��Bƿ�е�����Ϊ��SO2���壮��Cƿ��Ʒ����Һ����ɫ���ɵõ��Ľ�����SO2��������
��3��װ�â��м���Ĺ���ҩƷ����ˮCuSO4��Ŀ������֤��������к���ˮ������װ�â���ʢװ����Һ�dz���ʯ��ˮ��Ŀ������֤������к���CO2��
���� ��1���������������Ʒ����Һ�����������̼�ó����ʯ��ˮ������ˮ��������ˮ����ͭ������Һ����ˮ���������ȼ���ˮ��������ΪCO2���ó���ʯ��ˮ������ģ���SO2Ҳ����ʹ����ʯ��ˮ��룬����������ʹƷ����ɫ��������̼���ܣ����ԣ�������������ڼ��������̼֮ǰ����ͨ������ʯ��ˮȷ��CO2���ڣ�
��2������������ʹƷ����ɫ��SO2�ܺ���ˮ����������ԭ��Ӧ��
��3����ˮ����ͭ��ˮ���ã���ɫ����ɫ��������̼��ʹʯ��ˮ����ǣ�
��� �⣺��1���Ҵ���ŨH2SO4�¶ȹ��߷�Ӧ��CH3CH2OH+4H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$4SO2��+CO2��+7H2O+C��ѡ��װ�âܣ�����Һ����ˮ�����Ա����ȼ���ˮ������ѡ��װ�âۣ�������ˮ����ͭ��������˵����ˮ���ɣ��������̼�Ͷ���������ʹ�����ʯ��ˮ����ǣ�����������ʹƷ����ɫ��������̼���ܣ�������װ�â٣�Aװ��Ʒ����ɫ����˵����SO2���������Bװ������SO2��Cװ��Ʒ�첻��ɫ��˵��������ȫ�������װ�âڣ�ͨ������ʯ��ˮ����ǣ�ȷ��CO2���ڣ��ʴ�Ϊ���ܢۢ٢ڣ�
��2��ʵ��ʱװ�âٵ������Ǽ����������Ĵ��ڲ���ȥ��������Aƿ��װ��Ʒ����Һ������������ʹƷ����ɫ������������Ʒ����ɫ��˵�����������SO2��Bװ������SO2��SO2+Br2+2H2O=H2SO4+2HBr�������dz�ɫ��ȥ��Cװ��Ʒ�첻��ɫ��˵��SO2������ȫ���ʴ�Ϊ��Ʒ����ɫ�����������SO2����ɫ��ȥ����SO2���壻SO2��������
��3��װ�â۵������Ǽ���ˮ�������ð�ɫ����ˮ����ͭ��ĩ��CuSO4+5H2O�TCuSO4•5H2O����˵�������������ˮ������װ�âڵ������Ǽ��������̼���ó���ʯ��ˮ��Ca��OH��2+CO2=CaCO3��+H2O������ʯ��ˮ����ǣ�ȷ��CO2���ڣ��ʴ�Ϊ����ˮCuSO4��ˮ����������ʯ��ˮ��CO2��
���� ���⿼�����Ҵ���Ũ���ᷴӦ���ɶ�����������̼��ˮ������̿�ڲ���ļ��飬ע��ж��ֲ��������ʱ��Ӧ�����Ⱥ�˳������̼�Ͷ���������ʹ�����ʯ��ˮ����ǣ�����������ʹƷ����ɫ��������̼�����ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

 ��������������������ϵ�д�
��������������������ϵ�д� |  |  |  |
| �� | �� | �� | �� |
| A�� | ʵ����Թ����ջ����ˮ | |
| B�� | ʵ����Թ�����Һ��ΪѪ��ɫ | |
| C�� | ʵ����Թ��г���ש��ɫ���� | |
| D�� | ʵ���CuSO4��Һ�л��γ�һ��������ͨ· |
| A�� | ��ˮ��ˮϡ�ͺ���Һ��c ��NH3•H2O��/c ��NH4+����ֵ��С��c ��H+������ | |
| B�� | ��Ϊ�Ͻ��ڳ�ʪ�Ŀ��������γ�ԭ��أ����ԺϽ���ʴ�Զ��ϲ� | |
| C�� | ��ͬ�����£���Һ��Fe3+��Cu2+��Zn2+��������������ǿ | |
| D�� | ���������Ȼ�����Һ�����ղ��������������䣬���ù���Ϊ������ |
| A�� | pH=4��NaHSO3��Һ�У�c��Na+ ����c��HSO3-����c��H2SO3����c��SO32-�� | |
| B�� | 0.01mol•L-1 ��NaHCO3 ��Һ�д�������ƽ�⣺HCO3-?H++CO3 2-����ˮϡ����Һ�е�HCO3-��H+��CO3 2-Ũ�ȼ��� | |
| C�� | Ũ�Ⱦ�Ϊ0.1mol•L-1 ��CH3COOH��CH3COOK�����Һ�У�2c��H+��+c��CH3COOH��=2c��OH-��+c��CH3COO-�� | |
| D�� | ��Ka��HA��=3.6��10-4��Ka��HB��=1.75��10-5��������ʵ���Ũ�ȵ�NaA��KB��Һ��ȣ�c��Na+ ��-c��A-��=c��K+ ��-c��B-�� |
| A�� | 7�� | B�� | 8�� | C�� | 9�� | D�� | 10�� |
| ʵ�������� | �����¼ | ���۽��� | |
| A | ������Ũ������μ���Cu ��ϡ����Ļ������ | ��������ɫ���� | ���ᱻ��ԭΪNO2 |
| B | ������ǯ��ס�����ھƾ��� �ϼ��� | �����ۻ����������� | �۵㣺Al2 O3��Al |
| C | ��ij�Ȼ�������Һ�м��� Na2O2��ĩ | ���ֺ��ɫ���� | ����Na2O2��ĩǰ��ԭ�Ȼ� ������Һ�Ѿ����� |
| D | ����ɫʯ����Һ�г�����ʱ ��ͨ������ | ��Һ�ȱ�죬���� Ϊ��ɫ | ������Ư���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ƫ����泥�NH4VO3��Ϊ��ɫ����ɫ�ľ����ĩ������ˮ�Ͱ�ˮ������������ˮ���ڷ���ʪ��ұ����ռ��Ҫ��λ��
ƫ����泥�NH4VO3��Ϊ��ɫ����ɫ�ľ����ĩ������ˮ�Ͱ�ˮ������������ˮ���ڷ���ʪ��ұ����ռ��Ҫ��λ��