��Ŀ����
8��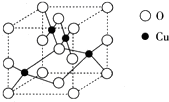 �ڢ��������������Se�����ڣ�Te����Ԫ���ڻ������г����ֳ����ּ�̬�����ڢ���Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣮
�ڢ��������������Se�����ڣ�Te����Ԫ���ڻ������г����ֳ����ּ�̬�����ڢ���Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣮��1����Ԫ�����ڱ���N��O��F����Ԫ�����ڣ�������Ԫ�صĵ�һ��������С�����˳����O��N��F��
��2��Seԭ�ӻ�̬������ӵ��Ų�ʽΪ1s22s22p63s23p63d104s24p4��CSe2�״�����H2Se��CCl4��Ӧ����ȡ�ģ�CSe2�����ڵ�Se-C-Se���Ǵ��ڣ�����ڡ������ڡ���С�ڡ���H2Se�����ڵ�H-Se-H�ļ��ǣ�
��3�����ݼ۲���ӶԻ������ۣ�������֪SO2���ӵĿռ乹��Ϊsp2������Sԭ�Ӳ��õĹ���ӻ���ʽΪV�Σ�
��4��ͭ������±�壨SCN��2��Ӧ����Cu��SCN��2����SCN��2�����ЦҼ���м��ĸ�����Ϊ5��4����±�壨SCN��2��Ӧ���������֣������������ᣨH-S-C��N���ķе�����������ᣨH-N=C=S���ķе㣬��ԭ��������������Ӽ���γ��������������Ӽ䲻���γ������
��5��ͭ�������γ�CuO��Cu2O���������ij��ͭ��������Ľṹ��ͼ��ʾ��
�ٸ�������Ļ�ѧʽΪCuO��
�ڸþ�������ԭ�ӵ���λ��Ϊ4�������о��������4��ͭԭ�ӹ��ɵļ�����״Ϊ�������壮
���� ��1��ͬ����Ԫ��������ң���һ�����ܳ��������ƣ���ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����һ�����ܴ���O��
��2������34��Ԫ�أ���̬ԭ�Ӻ�����34�����ӣ����ݹ���ԭ����д���������Ų�ʽ�����ݷ��ӿռ乹��ȷ���������Դ�С��
��3��������������У�����ԭ��S�γ�����2���Ҽ���1�Թ¶Ե��ӣ��ݴ��ж�������ԭ�ӵ����ʷ�ʽ���ռ乹�ͣ�
��4������[��SCN��2]�Ľṹ��֪��������3��������2��̼���μ�����������������Ӽ���γ������
��5�����ھ����ṹ�У�����ɫ�������Cuλ�����ڣ���4��������ɫ�������O��8��λ�ڶ��㡢6��λ�����ģ����þ�̯��������仯ѧʽ��
�ڸ��ݾ����ṹ��֪�����������ĵ�Oԭ����Χ����4�������ӣ���������λ��Ϊ4�������о��������4��ͭԭ�ӹ�����������ṹ��
��� �⣺��1��ͬ����Ԫ��������ң���һ�����ܳ��������ƣ����ǵ�ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������������ͣ����Ե�һ�����ܸ���ͬ��������Ԫ�صĵ�һ�����ܣ��������ߵĵ�һ��������С�����˳��Ϊ��O��N��F��
�ʴ�Ϊ��O��N��F��
��2������34��Ԫ�أ���̬ԭ�Ӻ�����34�����ӣ����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d104s24p4��CSe2��CO2�ṹ���ƣ�������̼��ֱ���ͷ��ӣ�H2Se����Ϊv�ͷ��ӣ�����CSe2�����ڵ�Se-C-Se���Ǵ���H2Se�����ڵ�H-Se-H���ǣ�
�ʴ�Ϊ��1s22s22p63s23p63d104s24p4�����ڣ�
��3��������������У�����ԭ��S�γ�����2���Ҽ�������1�Թ¶Ե��ӣ����ӻ������Ϊ3����ȡsp2�ӻ�����ռ�ṹΪV�Σ�
�ʴ�Ϊ��sp2��V�Σ�
��4������[��SCN��2]�Ľṹ��֪��������3��������2��̼�����μ������Է����ЦҼ��ͦм��ĸ�����Ϊ��3+2����4=5��4����������������Ӽ���γ��������������Ӽ䲻���γ���������������ᣨH-S-C��N���ķе�����������ᣬ
�ʴ�Ϊ��5��4������������Ӽ���γ��������������Ӽ䲻���γ������
��5�����ھ����ṹ�У�����ɫ�������Cuλ�����ڣ���4��������ɫ�������O��8��λ�ڶ��㡢6��λ�����ģ��������ܸ���Ϊ��8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ѧʽ��дΪCuO��
�ʴ�Ϊ��CuO��
������ͭ���������ĵ���������Χ��Χ��4��ͭ���ӣ�������λ��Ϊ4�����ھ����о��������4��ͭԭ�������ĵ���ԭ�Ӿ�����ȫ��ͬ���������ĸ�Cuԭ�ӹ�������������ṹ��
�ʴ�Ϊ��4���������壮
���� ���⿼���˾����ļ��㡢�����ijɼ�������жϡ�Ԫ�������ɵ�Ӧ�õ�֪ʶ����Ŀ�ѶȽϴ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ���������������ע�����վ�̯���ڼ��㾧����ѧʽ�е�Ӧ�ã���ȷ�����ijɼ������

 ������Һ��ʯ��������Ҫ�ɷ֣���ȼ��ʱ�ܷų��������ȣ���Ϊ��Դ�㷺Ӧ�����������ճ������У�
������Һ��ʯ��������Ҫ�ɷ֣���ȼ��ʱ�ܷų��������ȣ���Ϊ��Դ�㷺Ӧ�����������ճ������У���1����֪����2C3H8��g��+7O2��g���T6CO��g��+8H2O��l����H=-2 741.8kJ•mol-1
��2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1
��ӦC3H8��g��+5O2��g���T3CO2��g��+4H2O��l���ġ�H=-2219.9 kJ•mol-1��
��2��ij����С��Ϊ��̽������ͬ�¶��·�Ӧ�����������仯�뻯ѧƽ��Ĺ�ϵ����C3H8����ȫȼ�յIJ���CO��H2O��g����ͨ�뵽�����Ϊ1L��A��B�����ܱ������У�����ͬ�¶��¾��������¿��淴Ӧ��
CO��g��+H2O��g���TCO2��g��+H2��g����H=-41kJ•mol-1
����������£�
| ���� ��� | ��ʼʱ������ �����ʵ���/mol | �ﵽƽ�� ��ʱ��/min | �ﵽƽ��ʱ ��ϵ������ �仯/kJ | |||
| CO | H2O | CO2 | H2 | |||
| A | 1.5 | 1.9 | 0 | 0 | ta | �ų�������36.9 |
| B | 3 | 3.8 | 0 | 0 | tb | �ų�������Q |
��0��taʱ�̣�����A��CO��ƽ����Ӧ����Ϊ$\frac{0.9}{{t}_{a}}$ mol•L-1•min-1����ѧƽ�ⳣ��Ka=1.35��������B�з�Ӧ��ƽ�ⳣ��ΪKb������¶���Ka���ڣ�����ڡ���С�ڡ����ڡ���Kb��
��ijͬѧΪ���о���Ӧ�����Ի�ѧƽ���Ӱ�죬����淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��ͨ���о�ͼ���֣���Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬��t2��t8ʱ���ı���һ�����������Ǹı�������ֱ���t2�����¶ȣ�������CO2��Ũ�Ȼ�����H2��Ũ�ȣ���t8ʹ�ô������ѹ����С�������������
�۲��������A�з�Ӧ���е�t minʱ�����������CO2�����ʵ���Ϊ0.3mol������150mL 3mol•L-1��NaOH��Һ������ȫ���գ���������Һ������Ũ���ɴ�С��˳��Ϊc��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��3��CO2����ˮ����H2CO3����֪�������£�H2CO3��HClO�ĵ��볣�����£�
H2CO3?H++HCO${\;}_{3}^{-}$ Ka1=4.2��10-7 mol•L-1
HCO${\;}_{3}^{-}$?H++CO${\;}_{3}^{2-}$��Ka2=5.6��10-11 mol•L-1
HClO?H++ClO-��Ka=4.7��10-8 mol•L-1
��д��������̼���������ʵ���֮��Ϊ1��1ʱ������Ӧ�����ӷ���ʽ��Cl2+CO32-+H2O�TCl-+HClO+HCO3-��
| A�� | ����Ԫ���У�Y��ԭ�Ӱ뾶��� | |
| B�� | ����Ԫ���У�W����̬�⻯�����ȶ� | |
| C�� | Ԫ��W��X���γɺ��м��Լ��ļ��Է���WX3 | |
| D�� | ��ҵ�Ͽ��õ�����ڵ�Y��Z�Ļ�����ķ���ұ��Y��Z�ĵ��� |
���ǻ��ĵ���ʽ��
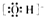
�ڴ�������ӵĽṹʽ��H-O-Cl
����ϩ�����ʽ��ʵ��ʽ����CH2=CH2
�ܺ���10�����ӵ���ԭ�ӣ�188O
�������ӵĽṹʾ��ͼ��
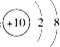
������̼���ӵı���ģ��

| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | ��������춡�黥Ϊͬ���칹�� | |
| B�� | �Ҵ������ᶼ��������������Һ��Ӧ | |
| C�� | ���ۺ͵����ʵ�ˮ����ﶼ�ǰ����� | |
| D�� | �����Ǻ����Ƕ��ܷ���ˮ�ⷴӦ |
| A�� | Ԫ��Z����Ԫ��X�γɹ��ۻ�����XZ2 | |
| B�� | Ԫ��X�����γɵ�ԭ�ӱ�Ϊ1��1�Ļ������кܶ��� | |
| C�� | Ԫ��Y�ĵ���������������Һ�����ᷴӦ������������ | |
| D�� | Ԫ��W��X���Ȼ����У���ԭ�Ӿ�����8���ӵ��ȶ��ṹ |
| A�� | �����÷���֬�Ʒ��� | |
| B�� | ��װʳƷ������ʯ�Ұ���ΪʳƷ�Ŀ������� | |
| C�� | ����CuSO4��Һʱ����������ϡ���� | |
| D�� | ���ƺ��ƵĻ���������ɫ��Ӧʵ�飬��ɫ��Ϊ��ɫ |
| A�� | lH��2H��3HΪͬ�������� | B�� | lH��2H��3H��Ϊͬλ�� | ||
| C�� | lH��2H��3H����������ͬ | D�� | lH��2H��3HΪͬ���칹�� |
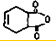 һ���������������������Ҫԭ�ϣ���ϳ�·�����£�
һ���������������������Ҫԭ�ϣ���ϳ�·�����£�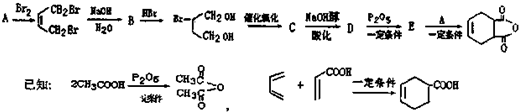
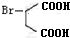 ��A��ϵͳ����Ϊ1��3-����ϩ��
��A��ϵͳ����Ϊ1��3-����ϩ�� ��
��