��Ŀ����
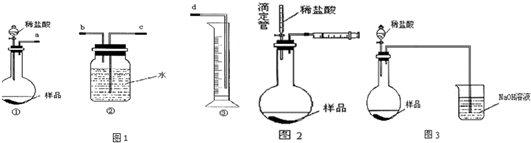
20����ҵ�����г���������NaCl��ijУ��ѧ����С�������ͼ��ʾװ�ã��ⶨ��ҵ������Na2CO3�ĺ�����
��1��Ҫ���鹤ҵ���������ʵĴ��ڣ��ȼ���B�����D�Լ���ѡ����ţ���
A������������Һ�� B��ϡ���ᡡ C�����軯����Һ�� D����������Һ
��2������װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ����©�������Լ�ƿ�е�Һ���ֲ��ٱ仯��©���е�Һ�治���½���˵��װ�ò�©����
��3��װ��A�������dz�ȥ������CO2��װ��C�е��Լ�ΪŨ���ᣮ
��4��ijͬѧ��Ϊ��Dװ�ú�Ӧ������Eװ�ã�װ���ʵ��Լ���������Ϊ�Ƿ��Ҫ����Ҫ��ѡ���Ҫ������Ҫ�������жϵ���������Ϊװ��E�����տ����еĶ�����̼��ˮ������Ӱ����������
��5��ʵ��ǰ��ȡ28.80g��Ʒ��ʵ�����Dװ������8.80g������Ʒ��Na2CO3����������Ϊ75%��
���� ��1�������г���������NaCl���ü��������ӵķ������м��飬��Ҫ�Լ�Ϊ��������ϡ���
��2������B�г���©�����ƿ��Һ��仯�����жϣ�
��3��װ��A��Ϊ�����տ����ж�����̼����Ӱ��̼���ƺ����IJⶨ��װ��C��Ũ����������ɵĶ�����̼���壬��Dװ�������պ���������أ�
��4��U�Թ������ն�����̼����������ⶨ̼���ƺ����ķ������������ֱ�ӽ�ͨ�������տ����ж�����̼��ˮ����Ӱ��ⶨ�����
��5��Dװ�����յ�Ϊ������̼���������̼Ԫ���غ���㣮
��� �⣺��1�������г���������NaCl���ü��������ӵķ������м��飬��Ҫ�Լ�Ϊ��������ϡ���ᣬ
A������������Һ ���ܼ��������Ӵ��ڣ���A��ѡ��
B��ϡ���� ��֤���ɵ��Ȼ������ܣ���Bѡ��
C�����軯����Һ�Ǽ��������ӵĴ��ڣ����ܼ��������ӣ���C��ѡ��
D����������Һ�������ӷ�Ӧ���ɰ�ɫ����������ϡ�����֤�����������ӣ���Dѡ��
�ʴ�Ϊ��BD��
��2������װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ������ѹǿ�仯��Һ��仯�����жϣ���©�������Լ�ƿ�е�Һ���ֲ��ٱ仯��֤��װ����������ã�
�ʴ�Ϊ��©�������Լ�ƿ�е�Һ���ֲ��ٱ仯��©���е�Һ�治���½���
��3��װ��A��Ϊ�����տ����ж�����̼������Ӱ��̼���ƺ����IJⶨ��װ��C��Ũ����������ɵĶ�����̼���壬��Dװ�������պ���������أ�
�ʴ�Ϊ����ȥ������CO2����ֹӰ����������Ũ���
��4��U�Թ������ն�����̼����������ⶨ̼���ƺ����ķ������������ֱ�ӽ�ͨ�������տ����ж�����̼��ˮ�����ⶨ���ƫ�ߣ�Ӱ��ⶨ�����ȷ�ԣ�
�ʴ�Ϊ����Ҫ����Ϊװ��E�����տ����еĶ�����̼��ˮ������Ӱ����������
��5��ʵ��ǰ��ȡ28.80g��Ʒ��ʵ�����Dװ������8.80g��Ϊ������̼���������̼Ԫ���غ���㣬��Ʒ��Na2CO3����������=$\frac{\frac{8.80g}{44g/mol}}{28.80g}$��106g/mol��100%=75%��
�ʴ�Ϊ��75%��
���� ���⿼�����Ʊ����ʵ�ʵ�鷽����ƺͺ����ⶨʵ�飬�������ʺͲⶨ�����еĸ��ŷ����ǽ���ؼ���ע��װ�õ����Ӻ��Լ������ã���Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �����жϷ�Ӧ�յ� | |
| B�� | �ڲⶨ��Ƥ�����ʱ����������Ƥ��ʵ������� | |
| C�� | �����жϷ�Ӧ�յ� | |
| D�� | п�������ܽ����Ƥδ��ɾ�ȥ���� |
| A�� | ���������� | B�� | �������ǽ��� | C�� | ����������� | D�� | �����ᡢ������ |
| A�� | �¶Ȳ��䣬���������������S2Cl2��ת���ʽ��� | |
| B�� | �¶Ȳ��䣬��С���������Һ�����ɫ���� | |
| C�� | ѹǿ���䣬�����¶ȣ�Һ�����ɫ��dz | |
| D�� | ������䣬�����¶ȣ�������ת���ʽ��� |
| ��� | ʵ��Ŀ�� | ʵ����� |
| A | ֤��Ksp��AgI����Ksp��AgCl�� | ��AgNO3��Һ�еμ�����NaCl��Һ��������ɫ�����������μ�����KI��Һ�ֲ�����ɫ���� |
| B | ��˾ƥ�ֵ��ᴿ������ȥ���е�ˮ����ۺ�������� | ����˾ƥ�ִ�Ʒ�ܽ�����������̼��������Һ�У����˺�����Һ�м������ᣬ�ٳ��˲�ϴ�� |
| C | ������ͷ�к���Ԫ�� | ���������ͷ����Һ�м�AgNO3��Һ��ϡ���� |
| D | �ӿ�п��ϡ���ᷴӦ��ȡH2������ | ��ϡ�����еμ�����Cu��NO3��2��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�����ṩ�ǻ������ṩ�ǻ��ϵ���ԭ�ӽ�ϳ�ˮ��
���ṩ�ǻ������ṩ�ǻ��ϵ���ԭ�ӽ�ϳ�ˮ��
������������ȷ���ǣ�������
| A�� | �١���������Ӧ�Ļ������Ǣ� | B�� | �١���������Ӧ�Ļ������Ǣ� | ||
| C�� | �ٵĻ����ǢڵĻ����Ǣ� | D�� | �ٵĻ����ǢڵĻ����Ǣ� |
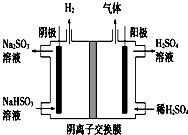 ��ҵ����Na2SO3����β���е�SO2��������ͼװ�õ�⣨���Ե缫��NaHSO3��ȡH2SO4�������缫��Ӧʽ2H++2e-=H2�����������ݳ�����ijɷ�ΪO2 SO2���ѧʽ����
��ҵ����Na2SO3����β���е�SO2��������ͼװ�õ�⣨���Ե缫��NaHSO3��ȡH2SO4�������缫��Ӧʽ2H++2e-=H2�����������ݳ�����ijɷ�ΪO2 SO2���ѧʽ����