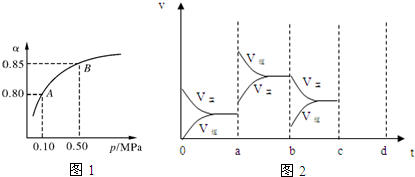
��Ŀ����
12����ϩ����Ҫ���л�����ԭ�ϣ���1����ϩ�����еĹ�������̼̼˫����д���ƣ���
��2��������ϩ�����ӳɷ�Ӧ��������ac����д��ţ���
a����ˮ b������ c���Ȼ��� d�����Ը��������Һ
��3����Ҫ�����������ϩ���ɽ����Ƿֱ�ͨ����ˮ������������Һ�����۲쵽��Һ��ɫ�����Ϊ��ϩ��
��4��ʵ������ȡ��ϩ�Ļ�ѧ����ʽΪCH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O����ӦҺ�м������Ƭ��Ŀ���Ƿ�ֹ���У�ŨH2SO4�������Ǵ�������ˮ����
���� ��1����ϩ�й�����Ϊ̼̼˫����
��2����ϩ����̼̼˫����������ˮ��HCl�����ӳɷ�Ӧ���ܱ����������Ը��������Һ������
��3����ϩ����̼̼˫���������巢���ӳɷ�Ӧ�������Ը��������Һ������ʹ��ˮ�����Ը��������Һ��ɫ�������鲻�ܣ�
��4��ʵ���������Ҵ���Ũ���ᡢ170�������·�����ȥ��Ӧ�Ʊ���ϩ����ӦҺ�м������Ƭ��Ŀ���Ƿ�ֹ���У�ŨH2SO4�������Ǵ�������ˮ����
��� �⣺��1����ϩ�Ľṹ��ʽΪCH2=CH2�����еĹ�����Ϊ̼̼˫�����ʴ�Ϊ��̼̼˫����
��2����ϩ����̼̼˫����������ˮ��HCl�����ӳɷ�Ӧ���ܱ����������Ը��������Һ��������ѡ��ac��
��3����ϩ����̼̼˫���������巢���ӳɷ�Ӧ�������Ը��������Һ������ʹ��ˮ�����Ը��������Һ��ɫ�������鲻�ܣ�������ͨ����ˮ������������Һ�����۲쵽��Һ��ɫ�����Ϊ��ϩ��
�ʴ�Ϊ����ˮ������������Һ������Һ��ɫ��
��4��ʵ���������Ҵ���Ũ���ᡢ170�������·�����ȥ��Ӧ�Ʊ���ϩ����Ӧ����ʽΪ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O����ӦҺ�м������Ƭ��Ŀ���Ƿ�ֹ���У�ŨH2SO4�������Ǵ�������ˮ����
�ʴ�Ϊ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O����ֹ���У���������ˮ����
���� ���⿼����ϩ�����ʡ��л��������ϩ���Ʊ��ȣ����ضԻ���֪ʶ���������գ��ѶȲ���

�ٰ����� �ڵ����� ��Al��OH��3 ��Al2O3 ��AlCl3 ��Al��
| A�� | �������� | B�� | �������� | C�� | �������� | D�� | ȫ�� |
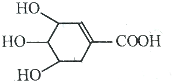 �˽����㺬��һ�ֿ����������в�������Ҫ�ɷ�--ç���ᣬ����ӽṹ��ͼ��ʾ�����й���ç�����˵��������ǣ�������
�˽����㺬��һ�ֿ����������в�������Ҫ�ɷ�--ç���ᣬ����ӽṹ��ͼ��ʾ�����й���ç�����˵��������ǣ�������| A�� | ����ͨ���ӳɷ�Ӧ��ȡ����Ӧ���ɺ���±ԭ�ӵIJ��� | |
| B�� | ����7����ԭ�� | |
| C�� | 1molç���������������Ʒ�Ӧ���Եõ�2mol���� | |
| D�� | �ܷ���������Ӧ��Ҳ�ܷ�����ȥ��Ӧ |
| A�� | 0.1mol/L�������pH=13������������Һ�������� | |
| B�� | 0.1mol/L��NaHCO3��Һ��pH=1������������� | |
| C�� | pH=3�������pH=11�İ�ˮ�������� | |
| D�� | pH=1�Ĵ����0.1mol/L������������Һ�������� |
| A�� | �������������С�ڷ�Ӧ��������� | |
| B�� | �÷�ӦΪ���ȷ�Ӧ | |
| C�� | �÷�ӦΪ���ȷ�Ӧ | |
| D�� | �÷�Ӧ��ÿ4 mol NH3��g�����������ų�905 kJ���� |
| A�� | Һ̬HCl����NaCl�������磬����HCl��NaCl ���Ƿǵ���� | |
| B�� | NH3��CO2��ˮ��Һ�����磬����NH3��CO2���ǵ���� | |
| C�� | ͭ ʯī�����磬�������Ǿ��ǵ���� | |
| D�� | ���ǡ��ƾ���ˮ��Һ������״̬�¾������磬�������Ǿ��Ƿǵ���� |
| A�� | 22 | B�� | ��ȷ�� | C�� | 22�� | D�� | 88�� |
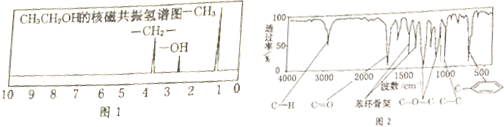
 ��
�� ��
�� ��
��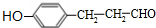 �ȣ�
�ȣ�