��Ŀ����
5�� ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺��1����Ũ��������ʵ���Ũ��Ϊ12mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����BD��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.3mol/Lϡ���ᣮ
�ٸ�ѧ����Ҫ��ȡ12.5 mL����Ũ����������ƣ�
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�BCAFED��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL����
�ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1-2cm��
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿���ƫ�ߡ���ƫ�͡�����Ӱ�족����
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ���ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ��
����Һע������ƿǰû�лָ������¾ͽ��ж��ݻ�ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ��
��4�����ڱ�״���£���VLHCl��������1Lˮ�У�������Һ�ܶ�Ϊd g/mL�������
Һ�����ʵ���Ũ��ΪABmol/L��
A.$\frac{1000Vd}{36.5V+22400}$ B.$\frac{1000Vd}{36.5V+22400}$ C.$\frac{1000Vd}{36.5V+22.4}$ D.$\frac{36.5V}{22.4}$
��5���ֽ�100mL 0.5mol/L��������200mL 0.1mol/LCuCl2��Һ��ϣ�����仯���Բ��ƣ�������Һ��Cl-�����ʵ���Ũ����0.3mol/L��
���� ��1������c=$\frac{1000�Ѧ�}{M}$�����Ũ������HCl�����ʵ���Ũ�ȣ�
��2�����ݸ��������Ƿ�����Һ������й��жϣ�
��3����������Һϡ���������ʵ����ʵ������������ҪŨ���������
����������һ�����ʵ���Ũ����Һ�IJ�������
�۸���c=$\frac{n}{V}$��������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС��V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ���V������ֵСʱ������ʹ������ҺŨ��ƫ��
��4������n=$\frac{V}{Vm}$�����Ȼ�����������ʵ������ٸ���m=nM����HCl������������m=��V����ˮ������������������Һ������������V=$\frac{m}{��}$������Һ�����������c=$\frac{n}{V}$�������������ʵ���Ũ�ȣ�
��5�������Һ������Ũ��=$\frac{n��}{V��}$���ݴ˽��
��� �⣺��1����������36.5%���ܶ�Ϊ1.2g/mL����������ʵ���Ũ��C=$\frac{1000��1.2��36.5%}{36.5}$=12mol/L��
�ʴ�Ϊ��12��
��2��A����Һ��HCl�����ʵ���=nV����������Һ������йأ���A��ѡ��
B����Һ���о�һ�ԣ���Һ��Ũ������Һ������أ���Bѡ��
C����Һ��Cl-����Ŀ=nNA=CVNA����������Һ������йأ���c��ѡ��
D����Һ���ܶ�����Һ������أ���Dѡ��
��ѡ��BD��
��3��������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�12mol/L��V=0.3mol/L��500mL�����V=12.5mL��
������һ�����ʵ���Ũ����Һ�IJ��裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ���ȷ�IJ���˳��Ϊ��BCAFED��
�ʴ�Ϊ��BCAFED��
�ۢ�����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ�������ƫС�������Ȼ�������ʵ���ƫС����ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ���ȴ����Һ���ƫС����ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��4��HCl�����ʵ���Ϊ$\frac{VL}{22.4L/mol}$=$\frac{V}{22.4}$mol��HCl������Ϊ$\frac{V}{22.4}$mol��36.5g/mol=$\frac{36.5V}{22.4}$g��1Lˮ������Ϊ1000mL��1g/mL=1000g������Һ������Ϊ��$\frac{36.5V}{22.4}$+1000��g����Һ�����Ϊ
$\frac{��\frac{36.5V}{22.4}+1000��}{1000dg/L}$=$\frac{36.5V+22400}{22400d}$L����������Һ�����ʵ���Ũ��Ϊ$\frac{\frac{\frac{V}{22.4}}{36.5V+22400}}{22400d}$=$\frac{1000dV}{36.5V+22400}$mol/L
��ѡ��AB��
��5���ֽ�100mL 0.5mol/L��������200mL 0.1mol/LCuCl2��Һ��ϣ�����仯���Բ��ƣ����Ϻ���Һ�������ӵ����ʵ���Ϊ��0.5mol/L��0.1L+0.1mol/L��2��0.2L=0.09mol��
�����Һ���V=0.1L+0.2L=0.3L��
����������Һ��Cl-�����ʵ���Ũ��=$\frac{0.09mol}{0.3L}$=0.3mol/L��
�ʴ�Ϊ��0.3mol/L��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��й����ʵ���Ũ�ȼ��㣬��ȷ����ԭ���Ͳ������̡���Ϥ�й����ʵ���Ũ�ȼ���Ĺ�ʽ���ɽ��ע������ƿ����Ͳ����ѡ��ע���������ķ�����

| A�� | 0.1mol/LNa2S��Һ������S2-����С��O.1NA | |
| B�� | ��CH3COONa��Һ��CH3COO-����ĿΪNA����Na+����Ŀ����NA | |
| C�� | һ�������£�1mol N2��3 mol H2��ϳ�ַ�Ӧ��ת�Ƶĵ�����ĿΪ6NA | |
| D�� | ��״���£�11.2 L�����к��еĻ�ѧ����ĿΪ9.5NA |
| A�� | ̽���¶ȶԷ�Ӧ����Ӱ��ʱ���ֱ�ˮԡ���������������Һ��������Һ��һ���¶ȣ��ٽ�����Һ��� | |
| B�� | H2O2�ڹ�������ø�Ĵ��£������¶ȵ����ߣ��ֽ����ʳ����ӿ� | |
| C�� | �ü������ȼƲⶨ��Ӧ�ȣ�ʹ������ĭ���ȱ��¡����β�����������衢��ȡ�ﵽ������¶ȣ����㷴Ӧ�ȣ�ȡ2��3 �ε�ʵ��ƽ��ֵ | |
| D�� | ȡ������Ӧ��Ļ��Һ������ˮ�У��۲������ж�������Ӧ�Ƿ���ȫ |
| A�� | $\frac{b-2c}{a}$mol��L-1 | B�� | $\frac{2b-c}{a}$mol��L-1 | C�� | $\frac{a-b}{a}$mol��L-1 | D�� | $\frac{2b-4c}{a}$mol��L-1 |
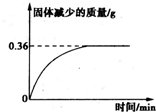 ������������½����������������ʯ���еľ�Ʒ���������£���ʯ���ֽ���
������������½����������������ʯ���еľ�Ʒ���������£���ʯ���ֽ���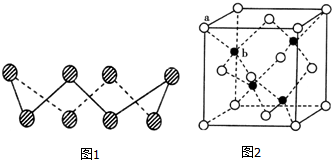 ��A�������������Se�����ڣ�Te����Ԫ���ڻ������г����ֳ���������̬������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
��A�������������Se�����ڣ�Te����Ԫ���ڻ������г����ֳ���������̬������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺