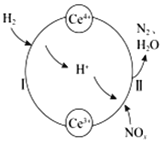
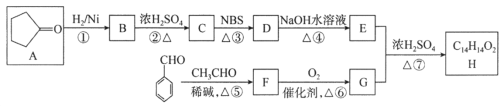
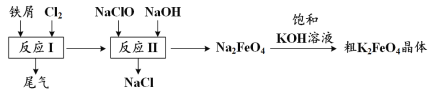��Ŀ����
����Ŀ��Cu��Zn���仯������������������������Ҫ���á�
��1��Cu��Zn�����ڱ���__������ɫ��ӦʱCu��4s���ӻ�ԾǨ��4p�����д��Cu�ļ���̬�����Ų�ʽ_��
��2���ֱ���CuSO4��MgSO4��Һ�Ӱ�ˮ��������ǰ��Ϊ����ɫ��Һ������Ϊ��ɫ������
��NH3��Cu2+�γ���������������Mg2+��ԭ����__��
����Һ�е�ˮ����H3O+��H5O2+������ʽ��H5O2+�ɿ�����H3O+��H2Oͨ������γɵ����ӣ���H5O2+�Ľṹʽ��__��
��3��Zn��ij�ֻ�����M�ǺܺõIJ�п�����ṹʽ��ͼ��
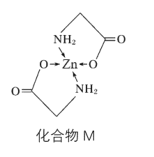
��1molM���е���������ĿΪ__��
�ڳ�����N��������H2NCH2COO-��NH3��N3-�ȣ�NH3�ķ��ӿռ乹��Ϊ__��N3-������Nԭ���ӻ���ʽΪ__��
��M�������������ʸߵ�ԭ�������п�γ���������__���������������������������������������������ά�����õ��ȶ��ԡ�
��4��±��п���۵������
±��п/ZnX2 | ZnF2 | ZnCl2 | ZnBr2 | ZnI2 |
�۵�/�� | 872 | 283 | 394 | 445 |
��ZnX2�۵�����仯��ԭ����__��
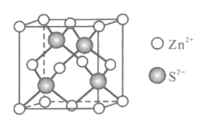
��5��Zn��ij������ľ�����ͼ��ʾ����֪�����ܶ�Ϊdgcm-3����S2-��Zn2+���У���Zn2+��S2+�ĺ˼��Ϊ__nm��д�������ʽ����
���𰸡�ds [Ar]3d104p1��1s22s22p63s23p63d104p1 ����Cu2+������NH3�ŵ��Ӷ���������������ص�����λ��ǿ������ͭ�����γ���λ���ܻ�ö����ȶ��ܣ��γɵ���λ�����ȶ�������·��˹��Ӳ������ۣ�ͭ���������ᣬ������������ ![]() 20NA ������ sp�ӻ� ���� �������Ͳ�ͬ��ZnF2Ϊ���Ӿ��塢ZnCl2��ZnBr2��ZnI2���Ӿ��壬��Ҫ�ƻ������Ӽ�ǿ�ȴ��ڷ��Ӽ�������
20NA ������ sp�ӻ� ���� �������Ͳ�ͬ��ZnF2Ϊ���Ӿ��塢ZnCl2��ZnBr2��ZnI2���Ӿ��壬��Ҫ�ƻ������Ӽ�ǿ�ȴ��ڷ��Ӽ������� ![]() ��
��![]() ��107
��107
��������
��1��CuΪ29��Ԫ�أ�λ�����ڱ��е�4���ڵڢ�B�壬Ϊds��Ԫ�أ�Zn�����ڱ���λ�ڵ�4���ڢ�B�壬����ds��Ԫ�أ�Cu�ĺ�������Ų�ʽΪ[Ar]3d104s1����Cu�ļ���̬�����Ų�ʽΪ��[Ar]3d104p1��1s22s22p63s23p63d104p1���ʴ�Ϊ��ds��[Ar]3d104p1��1s22s22p63s23p63d104p1��
��2��������Cu2+������NH3�ŵ��Ӷ���������������ص�����λ��ǿ���������ڽ�̩��ЧӦ������ЧӦ��ͭ�����γ���λ���ܻ�ö����ȶ��ܣ��γɵ���λ�����ȶ�����·��˹��Ӳ������ۣ�ͭ���������ᣬ������������ʴ�Ϊ������Cu2+������NH3�ŵ��Ӷ���������������ص�����λ��ǿ������ͭ�����γ���λ���ܻ�ö����ȶ��ܣ��γɵ���λ�����ȶ�������·��˹��Ӳ������ۣ�ͭ���������ᣬ�����������
��H5O2+�ɿ�����H3O+��H2Oͨ������γɵ����ӣ��ṹʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3����1��M�к���20����������M�к���20NA���������ʴ�Ϊ��20NA��
��NH3��Nԭ�ӳ�3����������һ��δ�ɼ��ŶԵ��ӣ��ӻ������Ϊ4����ȡsp3���ӻ����¶Ե��ӶԳɼ����ӵ��ų����ý�ǿ��N-H֮��ļ���С��109��28�������������ӿռ乹���������Σ�N3-��CO2��CS2��N2O��Ϊ�ȵ����壬������Nԭ���ӻ���ʽΪsp�ӻ���N3-��Nԭ�Ӽ۲���ӶԸ�������2�����ݼ۲���ӶԻ��������ж�Nԭ�ӵ��ӻ�������ͣ�Nԭ�Ӳ���sp�ӻ����ʴ�Ϊ�������ͣ�sp�ӻ���
��M�������������ʸߵ�ԭ�������п�γ��������ɱ��٣�������������ά�����õ��ȶ��ԣ��ʴ�Ϊ�����٣�
��4���ɱ����۵����ݿ�֪��ZnF2���������ֵľ������Ͳ�ͬ��ZnF2Ϊ���Ӿ��塢ZnCl2��ZnBr2��ZnI2���Ӿ��壬���Ӽ��ļ��ܴ��ڷ��Ӽ�����������ZnF2���۵�Զ������������±��п���ʴ�Ϊ���������Ͳ�ͬ��ZnF2Ϊ���Ӿ��塢ZnCl2��ZnBr2��ZnI2���Ӿ��壬��Ҫ�ƻ������Ӽ�ǿ�ȴ��ڷ��Ӽ���������
��5���þ�����S2-����Ϊ4��Zn2+����=8��![]() +6��
+6��![]() =4����S2-��Zn2+����֮��=4��4=1��1���þ����ⳤ=
=4����S2-��Zn2+����֮��=4��4=1��1���þ����ⳤ= ����ҪʹS2-��Zn2+���У�������Խ��߳���Ϊ2��п����ֱ����2��������ֱ��֮�ͣ�����Խ��߳���=
����ҪʹS2-��Zn2+���У�������Խ��߳���Ϊ2��п����ֱ����2��������ֱ��֮�ͣ�����Խ��߳���=![]() ��S2-�뾶��Zn2+�뾶֮��=
��S2-�뾶��Zn2+�뾶֮��=![]() ����Zn2+��S2+�ĺ˼��Ϊ
����Zn2+��S2+�ĺ˼��Ϊ![]() =
=![]() nm��
nm��
�ʴ�Ϊ��![]() ��
��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ��FeSO4��Һ�����ڿ��������ױ��ʣ����Ϊ�˷���ʹ��Fe2+��ʵ�����г�������������茶���[�׳ơ�Ħ���Ρ�����ѧʽΪ(NH4)2Fe(SO4)26H2O]�������̷����̷���Һ���ȶ���
I��ij��ȤС�����ʵ���Ʊ���������茶��塣
��ʵ���У�������Һ�Լ�����ʹ�õ�������ˮ��������С���ȴ����ʹ�á���FeSO4��Һ�м��뱥��(NH4)2SO4��Һ����������_______����ȴ�ᾧ�����ˡ�ϴ�Ӻ����õ�һ��dz����ɫ�ľ��塣
II��ʵ��̽��Ӱ����Һ��Fe2+�ȶ��Ե�����
(1)����0.8 mol/L��FeSO4��Һ��pH=4.5����0.8 mol/L��(NH4)2Fe(SO4)2��Һ��pH=4.0������ȡ2 mL������Һ����֧�Թ��У��տ�ʼ������Һ����dz��ɫ���ֱ�ͬʱ�μ�2��0.01mol/L��KSCN��Һ��15min��۲�ɼ���(NH4)2Fe(SO4)2��Һ��ȻΪdz��ɫ��������Һ��FeSO4��Һ����ֵ���ɫ���ǡ�
������1��
���� | Fe(OH)2 | Fe(OH)3 |
��ʼ���� pH | 7.6 | 2.7 |
��ȫ���� pH | 9.6 | 3.7 |
���������ӷ���ʽ����FeSO4��Һ��������ɫ���ǵ�ԭ��___________________��
������Ӱ��Fe2+�ȶ��Ե����أ�С��ͬѧ�������3�ּ��裺
����1������������ͬʱ��NH4+�Ĵ���ʹ(NH4)2Fe(SO4)2��Һ��Fe2+�ȶ��ԽϺá�
����2������������ͬʱ����һ�� pH��Χ�ڣ���Һ pHԽСFe2+�ȶ���Խ�á�
����3��__________________________________________________��
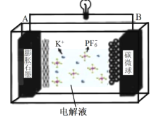
(2)С��ͬѧ����ͼװ�ã�GΪ���������ƣ�������������������Һ�ֱ������ҺA��0.2 mol/L NaCl������ҺB��0.1mol/L FeSO4��Ϊ��ͬ�� pH���۲��¼�����ƶ������Լ���2����ʵ���о���ʵ�������±���ʾ��
��� | A 0.2mol/LNaCl | B 0.1mol/LFeSO4 | �����ƶ��� |
ʵ��1 | pH=1 | pH=5 | 8.4 |
ʵ��2 | pH=1 | pH=1 | 6.5 |
ʵ��3 | pH=6 | pH=5 | 7.8 |
ʵ��4 | pH=6 | pH=1 | 5.5 |
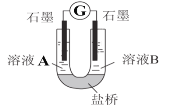
������2��ԭ���װ���У�����������ͬʱ��������Ӧ��Ļ�ԭ��Խǿ��������Ӧ���������Խǿ����ԭ��صĵ���Խ��
������3�������£�0.1mol/LpH=1��FeSO4��Һ��pH=5��FeSO4��Һ�ȶ��Ը��á�
��������ʵ������������Ϣ����С�����ۿ��Եó����½��ۣ�
��U������صĵ缫��Ӧʽ_________________��
�ڶԱ�ʵ��1��2����3��4������һ��pH��Χ�ڣ��ɵó��Ľ���Ϊ______ ��
�۶Ա�ʵ��_____��_____ ���ɵó���һ�� pH��Χ�ڣ���Һ����Ա仯�Ƕ�O2������ǿ����Ӱ�����ء�
�ܶԣ�����3��ʵ����ʵ�Ľ���Ϊ____________________��