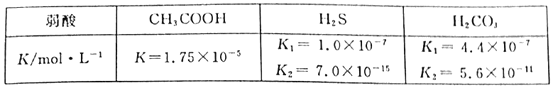��Ŀ����
����Ŀ��X��Y��Z��W��Q��R �����ڱ���ǰ 36 ��Ԫ�أ��˵���������������� X��Y��Z�� W ����Ԫ�����ڱ��ж�����Ԫ�ء�X Ϊ�ǽ���Ԫ�أ��� X ԭ�ӵĺ���ɶԵ�������δ�ɶԵ������� 2 ����Z �Ĵ�����������������������![]() ��W ԭ�ӵ� s ������ p ��������ȣ�Q ��ǰ�������е縺����С��Ԫ�أ�R ��ԭ������Ϊ 29�� �ش��������⣺
��W ԭ�ӵ� s ������ p ��������ȣ�Q ��ǰ�������е縺����С��Ԫ�أ�R ��ԭ������Ϊ 29�� �ش��������⣺
��1��X������������Ӧ��ˮ��������У�����ԭ�Ӳ�ȡ______________�ӻ���
��2�������� XZ �� Y �ĵ��ʷ��ӻ�Ϊ______________��1mol XZ �к�����������ĿΪ______________��
��3��W ���ȶ����Ӻ�����______________���˶�״̬�ĵ��ӡ�WԪ�صĵ�һ�����ܱ���ͬ���� ����Ԫ�صĵ�һ�����ܸߣ���ԭ������_____��
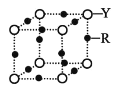
��4��Q �ľ���ṹ��ͼ��ʾ�����ڵ�λ������ Q ԭ�ӵĸ���Ϊ______________���������λ����______________��
��5��RԪ�صĻ�̬ԭ�ӵĺ�������Ų�ʽΪ________��Y �� R �γ�ij �ֻ�����ľ����ṹ��ͼ��ʾ����֪�þ�����ܶ�Ϊ��g��cm-3�������ӵ���������ֵΪ NA����þ����� R ԭ�Ӻ� Y ԭ��֮�����̾���Ϊ______________cm����ֻд����ʽ��
���𰸡�sp2 �ȵ����� 2NA 10 Mg ԭ�ӵļ۵����Ų�ʽΪ 3s2��3s �������ȫ��״̬���Ƚ��ȶ��� ʧȥһ�����ӱȽ����� 2 8 1s22s22p63s23p63d104s1 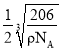
��������
X��Y��Z��W��Q��R �����ڱ���ǰ 36 ��Ԫ�أ��˵���������������� X��Y��Z�� W ����Ԫ�����ڱ��ж�����Ԫ�ء�X Ϊ�ǽ���Ԫ�أ��� X ԭ�ӵĺ���ɶԵ�������δ�ɶԵ������� 2 ������X�ĺ�������Ų�ʽΪ1s22s22p2����XΪ̼��Z �Ĵ�����������������������![]() ����ZΪ����YΪ����W ԭ�ӵ� s ������ p ��������ȣ���W�ĺ�������Ų�ʽΪ1s22s22p63s2��WΪþ��Q ��ǰ�������е縺����С��Ԫ�أ���QΪ�أ�R ��ԭ������Ϊ 29����RΪͭ���ݴ˷������
����ZΪ����YΪ����W ԭ�ӵ� s ������ p ��������ȣ���W�ĺ�������Ų�ʽΪ1s22s22p63s2��WΪþ��Q ��ǰ�������е縺����С��Ԫ�أ���QΪ�أ�R ��ԭ������Ϊ 29����RΪͭ���ݴ˷������
��1��X������������Ӧ��ˮ����ΪH2CO3���ṹʽΪ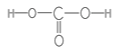 ��������ԭ��C��ȡsp2�ӻ����ʴ�Ϊ��sp2��
��������ԭ��C��ȡsp2�ӻ����ʴ�Ϊ��sp2��
��2��������CO��N2���Ӿ�����ͬ��ԭ�Ӹ������۲�����������ڵȵ����壻CO�Ľṹ��N2���ƣ�Ϊ![]() �����к�����������ĿΪ2NA���ʴ�Ϊ���ȵ����壻2NA��
�����к�����������ĿΪ2NA���ʴ�Ϊ���ȵ����壻2NA��
��3��Mg2+������10�����ӣ�����10���˶�״̬�ĵ��ӣ�WԪ�صĵ�һ�����ܱ���ͬ��������Ԫ�صĵ�һ�����ܸߣ���ԭ����Mg ԭ�ӵļ۵����Ų�ʽΪ 3s2��3s �������ȫ��״̬���Ƚ��ȶ���ʧȥһ�����ӱȽ����ѣ��ʴ�Ϊ��10��Mg ԭ�ӵļ۵����Ų�ʽΪ 3s2��3s �������ȫ��״̬���Ƚ��ȶ���ʧȥһ�����ӱȽ����ѣ�
��4�����ݾ����ṹ֪����ԭ���ڶ�������ģ����ڵ�λ�����м�ԭ�ӵĸ���Ϊ8��![]() +1=2�������ļ�ԭ������ļ�ԭ�Ӵ��ھ�����8�����㣬�������λ����8���ʴ�Ϊ��2��8
+1=2�������ļ�ԭ������ļ�ԭ�Ӵ��ھ�����8�����㣬�������λ����8���ʴ�Ϊ��2��8
��5��ͭ��̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1�������к���Nԭ����Ϊ��8��![]() =1�����е�Cuԭ����Ϊ��12��
=1�����е�Cuԭ����Ϊ��12��![]() =3����������Ϊ
=3����������Ϊ![]() ���辧�����ⳤΪd����d3=
���辧�����ⳤΪd����d3=![]() ����Rԭ�Ӻ�Yԭ��֮�����̾���Ϊ
����Rԭ�Ӻ�Yԭ��֮�����̾���Ϊ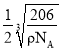 ���ʴ�Ϊ��1s22s22p63s23p63d104s1��
���ʴ�Ϊ��1s22s22p63s23p63d104s1��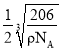 ��
��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�����Ŀ����ú����������ǿ��(����)�ɵõ���̿��ú���͡��ְ�ˮ�ͽ�¯����ij��¯��������£�
�ɷ� | ���� | ���� | ��ϩ | ��ϩ |
��������(%) | 20 | 56 | 14 | 10 |
���ý�¯��ͨ����������ˮ�����û�������ƽ����Է�������ԼΪ
A.5.63B.7.02C.7.41D.10.12
����Ŀ����1��ʵ����16 g�״�CH3OH(l)�������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�363.25 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ�� ____________________��
��2���ϳɰ���ӦN2(g)��3H2(g) ![]() 2NH3(g)��H��a kJ��mol��1����Ӧ���̵������仯��ͼ��ʾ��
2NH3(g)��H��a kJ��mol��1����Ӧ���̵������仯��ͼ��ʾ��
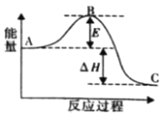
�ٸ÷�Ӧͨ���������������Ӵ�����ʹͼ��E______�����������������С������E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿______(��С����ޡ�)��
���йؼ����������£�
��ѧ�� | H��H | N��H | N��N |
����(kJ��mol��1) | 436 | 391 | 945 |
�Ը��ݱ������м������ݼ���aΪ____________��
��3����������ʱ�����£�N2H4��Ϊȼ�ϣ��ö�������Ϊ�����������������ʷ�Ӧ���ɵ�����ˮ��������֪����N2(g)+2O2(g)=2NO2(g) ����1=a kJ/mol
��N2H4(g)+O2(g)=N2(g)+2H2O(g) ����2=bkJ/mol
д���ºͶ���������Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ��_____________________��