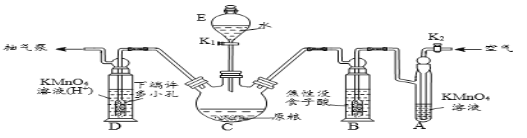
��Ŀ����
����Ŀ����1��ʵ����16 g�״�CH3OH(l)�������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�363.25 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ�� ____________________��
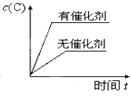
��2���ϳɰ���ӦN2(g)��3H2(g) ![]() 2NH3(g)��H��a kJ��mol��1����Ӧ���̵������仯��ͼ��ʾ��
2NH3(g)��H��a kJ��mol��1����Ӧ���̵������仯��ͼ��ʾ��
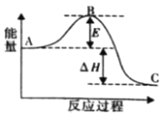
�ٸ÷�Ӧͨ���������������Ӵ�����ʹͼ��E______�����������������С������E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿______(��С����ޡ�)��
���йؼ����������£�
��ѧ�� | H��H | N��H | N��N |
����(kJ��mol��1) | 436 | 391 | 945 |
�Ը��ݱ������м������ݼ���aΪ____________��
��3����������ʱ�����£�N2H4��Ϊȼ�ϣ��ö�������Ϊ�����������������ʷ�Ӧ���ɵ�����ˮ��������֪����N2(g)+2O2(g)=2NO2(g) ����1=a kJ/mol
��N2H4(g)+O2(g)=N2(g)+2H2O(g) ����2=bkJ/mol
д���ºͶ���������Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ��_____________________��
���𰸡�CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=-726.5kJ/mol ��С �� -93 2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=(2b-a)kJ/mol
O2(g)=CO2(g)+2H2O(l) ��H=-726.5kJ/mol ��С �� -93 2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=(2b-a)kJ/mol
��������
��1��16 g�״������ʵ���=![]() ����ȼ�շų�363.25 kJ����������1mol�״���ȫȼ�շų�����Ϊ
����ȼ�շų�363.25 kJ����������1mol�״���ȫȼ�շų�����Ϊ![]() ������ȼ���ȵĶ��壬��ȼ�յ��Ȼ�ѧ��Ӧ����ʽΪ��CH3OH(l)+
������ȼ���ȵĶ��壬��ȼ�յ��Ȼ�ѧ��Ӧ����ʽΪ��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=-726.5kJ/mol��
O2(g)=CO2(g)+2H2O(l) ��H=-726.5kJ/mol��
��2���ٴ����ܽ��ͷ�Ӧ�����ܣ�����Ӱ���ʱ�Ĵ�С���ʴ�Ϊ����С���ޣ�
����H=��Ӧ����ܼ���֮��-��������ܼ���֮�ͣ���![]() ���ʴ�Ϊ��93��
���ʴ�Ϊ��93��
��3�����ø�˹���ɣ�![]() ���ɵõ�Ŀ���Ȼ�ѧ��Ӧ����ʽ����2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=(2b-a)kJ/mol��
���ɵõ�Ŀ���Ȼ�ѧ��Ӧ����ʽ����2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ��H=(2b-a)kJ/mol��