��Ŀ����
A��B��C����Ԫ�ص�ԭ�Ӿ�����ͬ�ĵ��Ӳ�������B�ĺ˵������A��2��Cԭ�ӵĵ���������Bԭ�ӵĵ���������4�� 1molA�ĵ����ܸ����ᷴӦ���ڱ�״���¿��û���11.2L��H2����ʱAת��Ϊ����ԭ�Ӿ�����ͬ���Ӳ�ṹ�����ӣ��Իش�
��1��A��
��2��B�����ӽṹʾ��ͼ��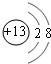
 ����B�����Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ���Σ��÷�Ӧ�Ļ�ѧ����ʽ��
����B�����Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ���Σ��÷�Ӧ�Ļ�ѧ����ʽ��
��3��A��CԪ���γɻ�����ĵ���ʽ��
 �����ڱ�����C�������ڵ�ͬ����Ԫ���γɵ���̬�⻯���У��ȶ�����ǿ����
�����ڱ�����C�������ڵ�ͬ����Ԫ���γɵ���̬�⻯���У��ȶ�����ǿ����
��4��д��A��B��������������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ��
��1��A��
Na
Na
Ԫ�أ�B��Al
Al
Ԫ�أ�C��Cl
Cl
Ԫ�أ���2��B�����ӽṹʾ��ͼ��


NH3+HCl�TNH4Cl
NH3+HCl�TNH4Cl
����3��A��CԪ���γɻ�����ĵ���ʽ��


HF
HF
�����⻯�ﻯѧʽ������4��д��A��B��������������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ��
Al��OH��3+OH-=AlO2-+2H2O
Al��OH��3+OH-=AlO2-+2H2O
��������A��B��C����Ԫ�ص�ԭ�Ӿ�����ͬ�ĵ��Ӳ�������B�ĺ˵������A��2��Cԭ�ӵĵ���������Bԭ�ӵĵ���������4��1molA�ĵ����ܸ����ᷴӦ���ڱ�״���¿��û���11.2L��H2�����������ʵ�����0.5mol������ת�Ƶ������֪��A�ǵ�IA��Ԫ�أ���ʱAת��Ϊ����ԭ�Ӿ�����ͬ���Ӳ�ṹ�����ӣ���A��NaԪ�أ�B��AlԪ�أ�C��ClԪ�أ�
����⣺A��B��C����Ԫ�ص�ԭ�Ӿ�����ͬ�ĵ��Ӳ�������B�ĺ˵������A��2��Cԭ�ӵĵ���������Bԭ�ӵĵ���������4��1molA�ĵ����ܸ����ᷴӦ���ڱ�״���¿��û���11.2L��H2�����������ʵ�����0.5mol������ת�Ƶ������֪��A�ǵ�IA��Ԫ�أ���ʱAת��Ϊ����ԭ�Ӿ�����ͬ���Ӳ�ṹ�����ӣ���A��NaԪ�أ�B��AlԪ�أ�C��ClԪ�أ�
��1��ͨ�����Ϸ���֪��A��B��C�ֱ���Na��Al��Cl���ʴ�Ϊ��Na��Al��Cl��
��2��B�����������ӣ���2�����Ӳ㣬�������8�����ӣ����������ӽṹʾ��ͼΪ�� ����B�����Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ���Σ���÷����ǰ������ӣ����������ᷴӦ�����Ȼ�泥����Ը÷�Ӧ�Ļ�ѧ����ʽ NH3+HCl�TNH4Cl���ʴ�Ϊ��
����B�����Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ���Σ���÷����ǰ������ӣ����������ᷴӦ�����Ȼ�泥����Ը÷�Ӧ�Ļ�ѧ����ʽ NH3+HCl�TNH4Cl���ʴ�Ϊ�� ��NH3+HCl�TNH4Cl��
��NH3+HCl�TNH4Cl��
��3��A��C���γɵĻ��������Ȼ��ƣ������ʽ�� ��Ԫ�صķǽ�����Խǿ������̬�⻯��Խ�ȶ���ͬһ����Ԫ���У�Ԫ�صķǽ���������ԭ���������������С�����ڱ�����C�������ڵ�ͬ����Ԫ���γɵ���̬�⻯���У��ȶ�����ǿ����HF��
��Ԫ�صķǽ�����Խǿ������̬�⻯��Խ�ȶ���ͬһ����Ԫ���У�Ԫ�صķǽ���������ԭ���������������С�����ڱ�����C�������ڵ�ͬ����Ԫ���γɵ���̬�⻯���У��ȶ�����ǿ����HF��
�ʴ�Ϊ�� ��HF��
��HF��
��4��A������������ˮ�������������ƣ�B������������ˮ�������������������������ܺ�ǿ�Ӧ�����κ�ˮ�����������ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��1��ͨ�����Ϸ���֪��A��B��C�ֱ���Na��Al��Cl���ʴ�Ϊ��Na��Al��Cl��
��2��B�����������ӣ���2�����Ӳ㣬�������8�����ӣ����������ӽṹʾ��ͼΪ��
 ����B�����Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ���Σ���÷����ǰ������ӣ����������ᷴӦ�����Ȼ�泥����Ը÷�Ӧ�Ļ�ѧ����ʽ NH3+HCl�TNH4Cl���ʴ�Ϊ��
����B�����Ӿ�����ͬ�����������У���һ�ַ��ӿ������ữ������һ���Σ���÷����ǰ������ӣ����������ᷴӦ�����Ȼ�泥����Ը÷�Ӧ�Ļ�ѧ����ʽ NH3+HCl�TNH4Cl���ʴ�Ϊ�� ��NH3+HCl�TNH4Cl��
��NH3+HCl�TNH4Cl����3��A��C���γɵĻ��������Ȼ��ƣ������ʽ��
 ��Ԫ�صķǽ�����Խǿ������̬�⻯��Խ�ȶ���ͬһ����Ԫ���У�Ԫ�صķǽ���������ԭ���������������С�����ڱ�����C�������ڵ�ͬ����Ԫ���γɵ���̬�⻯���У��ȶ�����ǿ����HF��
��Ԫ�صķǽ�����Խǿ������̬�⻯��Խ�ȶ���ͬһ����Ԫ���У�Ԫ�صķǽ���������ԭ���������������С�����ڱ�����C�������ڵ�ͬ����Ԫ���γɵ���̬�⻯���У��ȶ�����ǿ����HF���ʴ�Ϊ��
 ��HF��
��HF����4��A������������ˮ�������������ƣ�B������������ˮ�������������������������ܺ�ǿ�Ӧ�����κ�ˮ�����������ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
���������⿼����λ�ýṹ�����ʵ����ϵ����ȷ�ƶ�Ԫ���ǽⱾ��ؼ����������������������ӷ�Ӧ����ʽ����д�dz�����㣬Ҳ���״��㣮

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ

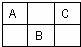 ��ͼ�����ڱ��ж����ڵ�һ���֣�A��B��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������2��������Ԫ�ط��ű�ʾ��
��ͼ�����ڱ��ж����ڵ�һ���֣�A��B��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������2��������Ԫ�ط��ű�ʾ��