��Ŀ����
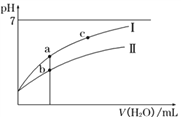
����Ŀ����֪��25��ʱijЩ����ĵ���ƽ�ⳣ��������ͼ���ʾ������ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯������˵����ȷ���ǣ� ��

A. ��ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȴ�С��ϵΪ��c(Na+) �� c(ClO-) �� c(CH3COO-)�� c(OH-) �� c(H+)
B. ��NaClO��Һ��ͨ����CO2�����ӷ���ʽΪ��2ClO- + CO2 + H2O = 2HClO + CO32-
C. ͼ����a��c���㴦����Һ��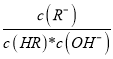 ��ȣ�HR����CH3COOH��HClO��
��ȣ�HR����CH3COOH��HClO��
D. ͼ����a���Ӧ�����Ũ�ȴ���b�������Ũ��
���𰸡�C
��������A������ĵ��볣�����ڴ����ᣬ���Դ������ˮ��̶�С�ڴ�������������ƺʹ������ƶ���ǿ�������Σ�������Һ�ʼ��ԣ�������ͬ���ʵ���Ũ�ȵ�CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ�ǣ�c(Na+)��c(CH3COO-)��c(ClO-)��c(OH-)��c(H+)����A����B��̼��Ķ�������С�ڴ����ᣬ����̼��������ӵ�����С�ڴ����ᣬ����NaClO��Һ��ͨ������������̼�����ӷ���ʽ��ClO-+CO2+H2O�THClO+HCO3-����B����C����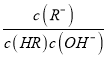 �ķ��ӡ���ĸͬʱ����������Ũ�ȿɵã�
�ķ��ӡ���ĸͬʱ����������Ũ�ȿɵã� ![]() ������ˮ�����ӻ��͵���ƽ�ⳣ��ֻ���¶�Ӱ�죬a��c���¶���ͬ����ñ�ֵ��ȣ���C��ȷ��D��pH��ȵ�CH3COOH��HClO��ϡ����ͬ�ı���ʱ����ǿ����������Ũ��С�ڽ����ᣬ��������pHС�ڽ�ǿ�ᣬ����CH3COOH��HClO������a�������߱�ʾCH3COOH��b�������߱�ʾHClO��������ĵ���̶�С�ڴ��ᣬ���Դ����Ũ�Ƚ�С���������Ũ�Ƚϴ�a��b������ȣ�������ͬ�����ˮ����Ȼ�Ǵ������Ũ�Ƚϴ�ͼ����a�����Ũ��С��b�����Ũ�ȣ���D��������ѡC��
������ˮ�����ӻ��͵���ƽ�ⳣ��ֻ���¶�Ӱ�죬a��c���¶���ͬ����ñ�ֵ��ȣ���C��ȷ��D��pH��ȵ�CH3COOH��HClO��ϡ����ͬ�ı���ʱ����ǿ����������Ũ��С�ڽ����ᣬ��������pHС�ڽ�ǿ�ᣬ����CH3COOH��HClO������a�������߱�ʾCH3COOH��b�������߱�ʾHClO��������ĵ���̶�С�ڴ��ᣬ���Դ����Ũ�Ƚ�С���������Ũ�Ƚϴ�a��b������ȣ�������ͬ�����ˮ����Ȼ�Ǵ������Ũ�Ƚϴ�ͼ����a�����Ũ��С��b�����Ũ�ȣ���D��������ѡC��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�



