��Ŀ����
1�����ɷ��Ӿ����������__________���������Ӽ��������һ����__________����������__________��������Ӽ�������ֻ��__________������һ������Ϊ���ģ�����Χͨ��������__________�����ڵķ��ӣ����Ӿ������һ������Ϊ__________���ɱ���CO2���Ӳ�ȡ�ľ��������Ķѻ���ʽ���������ڴ�����������������ǿ���뾶��С��__________ԭ����Hԭ�����γɵĹ��ۼ�������γ����������������б����ԣ�Ҳ����__________�����γ���������ʽ϶࣬��______________________________�ȡ��ڱ��У�ÿ��ˮ���������ڵ�__________��ˮ�����γ��������__________����ṹ��
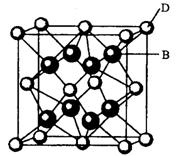
��2����ԭ�Ӿ����У�����ԭ�Ӷ���__________����ϣ�����ԭ�Ӿ�����һ����ά��__________�ṹ���ڽ��ʯ�У�Cԭ�Ӳ�ȡ__________�ӻ���ʽ��ÿ��Cԭ��������__________��Cԭ���Թ��ۼ����ϣ�����Ϊ__________����������С̼����__________��Cԭ�ӹ��ɣ��Ҳ���ͬһ��ƽ���ڡ������Ľṹ����ʯ���ƣ�SiO2�ľ���ṹ���Կ������ھ�����Si��Si��֮�����__________ԭ�Ӷ��γɵģ���12 g SiO2�����к��л�ѧ�������ʵ���Ϊ __________mol��
��2����ԭ�Ӿ����У�����ԭ�Ӷ���__________����ϣ�����ԭ�Ӿ�����һ����ά��__________�ṹ���ڽ��ʯ�У�Cԭ�Ӳ�ȡ__________�ӻ���ʽ��ÿ��Cԭ��������__________��Cԭ���Թ��ۼ����ϣ�����Ϊ__________����������С̼����__________��Cԭ�ӹ��ɣ��Ҳ���ͬһ��ƽ���ڡ������Ľṹ����ʯ���ƣ�SiO2�ľ���ṹ���Կ������ھ�����Si��Si��֮�����__________ԭ�Ӷ��γɵģ���12 g SiO2�����к��л�ѧ�������ʵ���Ϊ __________mol��
��1�����ӡ����»�������������»���12�������ܶѻ���N��O��F�������ԡ�HF��NH3��H2O��CH3OH�ȡ�4����������
��2�����ۡ�������״��sp3��4��109��28�䡡6������0.8
��2�����ۡ�������״��sp3��4��109��28�䡡6������0.8
���⿼���˷����ܶѻ���������Լ����͵ķ��Ӿ����ԭ�Ӿ���Ľṹ����SiO2������ÿһ����ԭ�Ӷ�����Χ���ĸ���ԭ���γ����ĸ������������Ҳ����ڹ��õ����������SiO2�����л�ѧ������ĿΪ��ԭ����Ŀ���ı���12 g SiO2��������ʵ�����0.2 mol�����Ի�ѧ�������ʵ���Ϊ0.8 mol��

��ϰ��ϵ�д�
�����Ŀ
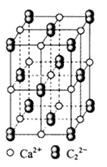
 �����ж�CA3����ˮ���γ�CA3��H2O�ĺ����ṹ ������ĸ���ţ������������ǣ�
�����ж�CA3����ˮ���γ�CA3��H2O�ĺ����ṹ ������ĸ���ţ������������ǣ�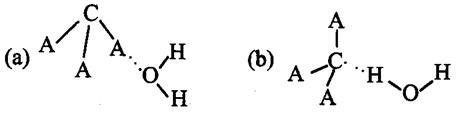

 ��ˮ��Ӧ������Ȳ��
��ˮ��Ӧ������Ȳ��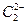 ��
��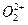 ��Ϊ�ȵ����壬
��Ϊ�ȵ����壬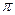 ����ĿΪ ��
����ĿΪ ��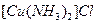 ��Һ����
��Һ����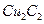 ����ɫ������
����ɫ������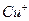 ��̬��������Ų�ʽΪ ��
��̬��������Ų�ʽΪ ��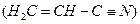 ����ϩ�������̼ԭ�ӹ���ӻ������� �������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ ��
����ϩ�������̼ԭ�ӹ���ӻ������� �������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ ��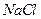 �������ƣ���ͼ��ʾ������
�������ƣ���ͼ��ʾ������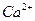 ��Χ���������
�����������
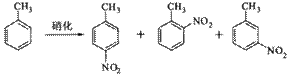
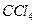 ��Һ�У�����������������ˮ���ã���45�淴Ӧ1h ����Ӧ�������ˣ���Һ�ֱ���5% NaHCO3,��Һ��ˮϴ�����ԣ��پ������ᴿ�õ��������ױ���
��Һ�У�����������������ˮ���ã���45�淴Ӧ1h ����Ӧ�������ˣ���Һ�ֱ���5% NaHCO3,��Һ��ˮϴ�����ԣ��پ������ᴿ�õ��������ױ��� NaHSO4���Ʊ��������ױ�ʱ��������ױ���������ʵ���֮��Ϊ ��
NaHSO4���Ʊ��������ױ�ʱ��������ױ���������ʵ���֮��Ϊ ��