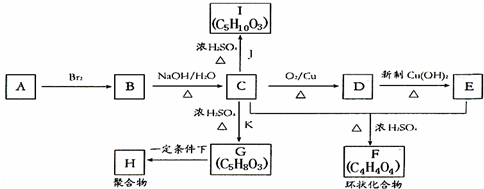
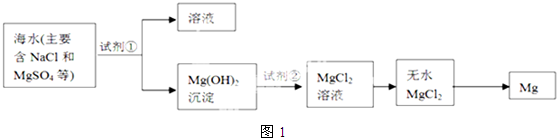
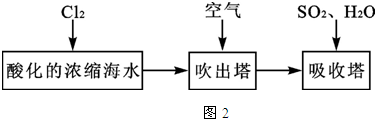
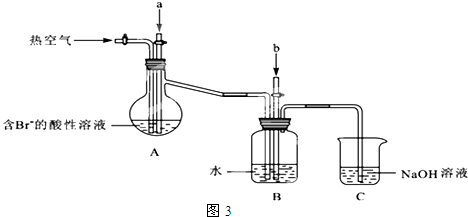
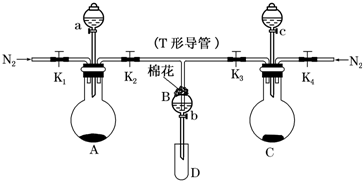
��Ŀ����
6��ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش���������
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����BD������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������500mL����ƿ ��������
��2��������ƿ��ʹ�÷����У����в�������ȷ����BC������ţ�
A��ʹ������ƿǰ�����Ƿ�©ˮ
B������NaOH��Һʱ���ѳƺõ�NaOH������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߣ�
C������H2SO4��Һʱ������ƿ������ˮϴ����Ҫ��0.5mol/L H2SO4��Һ��ϴ������ʹ�ã�
D���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ�
��3�����ݼ�����������ƽ��ȡ��NaOH��������Ϊ2.0g��
��4�����ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ13.6 mL ������1λС������
��5������������Һ�����У����в�����������ƫ�ߵ���B������ţ�
A��δϴ���ձ���������
B��δ��ȴ�����¾�ת�Ƶ�����ƿ����
C������ʱ�������ӿ̶��ߣ�
���� ��1����������һ�����ʵ���Ũ����Һ����ѡȡ������
��2����������ƿ�Ĺ��켰��ȷʹ�÷������н��
��3������m=CVM������Ҫ���ʵ�������
��4���ȸ���C=$\frac{1000�Ѧ�}{M}$����Ũ�����Ũ�ȣ��ٸ���Ũ����ϡ��ǰ�����ʵ����ʵ���������㣻
��5�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������һ�����ʵ���Ũ����Һ�õ������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����Ҫ�������У�ƽ����ƿ�ͷ�Һ©������ͼ�����������⣬����������Һ����Ҫ�IJ���������500mL����ƿ ��������
�ʴ�Ϊ��BD��500mL����ƿ ��������
��2��A������ƿʹ��ǰӦ����Ƿ�©ˮ������ȷ��
B������ƿΪ�������������������ܽ�������ϡ����Һ����B����
C������H2SO4��Һʱ������ƿ������ˮϴ����Ҫ��0.5mol/L H2SO4��Һ��ϴ���������ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���C����
D�����ݽ�������Ҫ����ҡ�ȣ���������Ϊ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ���D��ȷ��
��ѡ��BC��
��3��Ҫ����0.1mol/L NaOH��Һ480mL��Ӧѡ��500mL����ƿ����Ҫ������������=0.1mol/L��0.5L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��4��Ũ�����Ũ��C=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=0.5/L��0.5L��V=0.0136L=13.6mL��
�ʴ�Ϊ��13.6��
��5��A��δϴ���ձ������������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A��ѡ��
B��δ��ȴ�����¾�ת�Ƶ�����ƿ���ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���Bѡ��
C������ʱ�������ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���C��ѡ��
��ѡ��B��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�ѶȲ�����ȷ���Ʋ���Ϊ���ؼ���ע���������ƹ������������ķ����뼼�ɣ���������������ѧ���Ļ�ѧʵ��������


| A�� | ��Ӧ���̿ɱ�ʾΪ | |
| B�� | E1Ϊ��Ӧ���ƽ�����������̬���������Ϊ����Ӧ�Ļ�� | |
| C�� | ��ͼ���淴Ӧ����ЧӦ��H=E1-E2��Ϊ���ȷ�Ӧ | |
| D�� | ��ͼ������Ӧ����ЧӦΪ��H=E1-E2����E2��E1����������ӦΪ���ȷ�Ӧ |
| A�� | CH4+Cl2$\stackrel{��}{��}$CH3Cl+HCl | |
| B�� |  +Br2$\stackrel{����}{��}$ +Br2$\stackrel{����}{��}$ +H2O +H2O | |
| C�� | 2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O | |
| D�� | CH2�TCH2+HCl��CH3CH2Cl |
| A�� | �����ܽ���ˮ��Cl2+H2O�THClO+Cl-+H+ | |
| B�� | ��ˮ�е��뱥��FeCl3��Һ����Һ�ʺ��ɫ��Fe3++3H2O�TFe��OH��3��+3H+ | |
| C�� | ��������Һ�Լ��ԣ�CH3COO-+H2O�TCH3COOH+OH-�� | |
| D�� | ���õ�H2S ��Һ����ǣ�2S2-+O2+4H+�T2S��+2H2O |

| A�� | �Ҵ� | B�� | Ũ���� | C�� | NaCl | D�� | HCl |