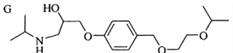
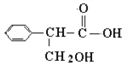
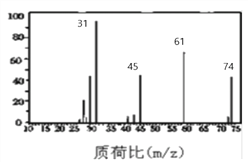
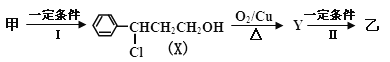��Ŀ����
����Ŀ����ͼ��AΪ278g/mol��B��D��E��F��G����������BΪ����ɫ���壬F��K���⻯�C��H���ճ�����������Ľ������ʣ�J�ǻ���ɫ���塣O�ǰ�ɫ������ͼ�в��ַ�Ӧ���������û���г�����

��1��д��A��G��L�Ļ�ѧʽA______________��G�� _______________��L��______________��
��2����Ӧ�ڵĻ�ѧ����ʽ ________________________________________________��
��3��д����ӦM��L�����ӷ���ʽΪ________________________________________��
��4������O���ھ��ã����ʹ����е�����Ϊ___________________________________�������Ļ�ѧ����ʽΪ__________________________________________________��
��5����M��Һ��Ͷ����M�����ʵ�����Na2O2����Ӧ�����ӷ���ʽ��____________��
���𰸡�FeSO4��7H2OAl2O3FeCl3SO2+Cl2+2H2O=4H2SO4+2HCl2Fe2++Cl2=2Fe3++2Cl-��ɫ�����ɫ����ɫ4Fe(OH)2+O2+2H2O=4 Fe(OH)34Na2O2 + 4Fe2+ + 6H2O = 8Na+ + O2�� + 4 Fe(OH)3��
��������
ץסB��D��E��F��G�������F��K���⻯�C��H���ճ�����������Ľ������ʣ�B + C��G + H��Ӧ�������Ǹ��£��������뵽�����ȷ�Ӧ��C���� G����������K + H ��M��K���⻯�O�ǰ�ɫ������B��H��L��M��N��O�к���ͬ��Ԫ�أ��Լ�J+H��L��M+J��L��L��N��M��O��O��N��N��B���ݹ�ϵ���Զ϶�B��H��L��M��N��O�к���ͬ��Ԫ����Fe ����DΪ��������EΪ��������FΪˮ��HΪ����IΪ���ᡢJΪ������KΪ���ᡢLΪ�Ȼ�����MΪ�Ȼ�������OΪ������������NΪ����������BΪ����ɫ������Ϊ��������AΪ278g/mol�ۺϷ���֪��A����ˮ���������������Ԣ�A��G��L�Ļ�ѧʽA��FeSO4��7H2O��G��Al2O3��L��FeCl3���Ʒ�Ӧ���Ƕ���������������ˮ��Ӧ������������ᣬ��Ӧ�����ӷ���ʽΪSO2+Cl2+2H2O=4H2SO4+2HCl���Ƿ�ӦM��L���Ȼ�������������Ӧ�����Ȼ�������Ӧ�����ӷ���ʽΪ2Fe2++Cl2=2Fe3++2Cl-����OΪ���������������ھ��ã������ᱻ�����е��������������������������������������ʱ��ʹ����е�����Ϊ��ɫ�����ɫ����ɫ�������Ļ�ѧ����ʽΪ4Fe(OH)2+O2+2H2O=4 Fe(OH)3����5��MΪ�Ȼ���������M��Һ��Ͷ����M�����ʵ�����Na2O2����Ӧ�����ӷ���ʽ��4Na2O2 + 4Fe2+ + 6H2O = 8Na+ + O2�� + 4 Fe(OH)3����

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ����1���±��ո��е�������ʽΪ_______������_______��ͬ���칹�塣
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
CH4 | C2H4 | C3H8 | C4H8 | C6H12 | C7H16 | C8H16 |
��2��������(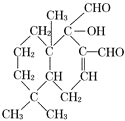 )��һ������ɱ������京�������ŵ�����Ϊ_____________________��
)��һ������ɱ������京�������ŵ�����Ϊ_____________________��
��3��CH2=CH-![]() -C��C-CH3�У������_______��ԭ�ӹ��档
-C��C-CH3�У������_______��ԭ�ӹ��档
��4���ٸ���������д�ṹ��ʽ��2-��-2,4-�Ѷ�ϩ_________________________________
��5���л���![]() ��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��ϵͳ������___________________________________�������ڴ�����������ȫ�⻯������������ϵͳ������_________________________________
��6��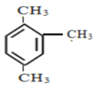 ��ϵͳ������______________________________
��ϵͳ������______________________________