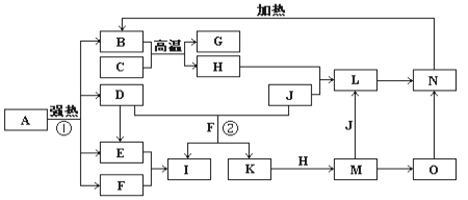
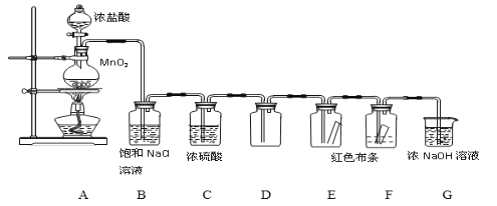
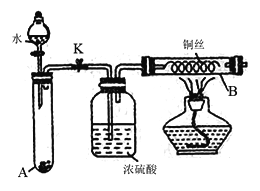
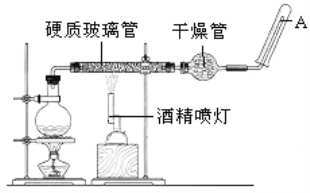
��Ŀ����
����Ŀ����1��ȡ3.7gij�л���A�����������г��ȼ�գ�ֻ����8.8 gCO2��4.5g H2O����A�к��е�Ԫ��Ϊ_________________����Ԫ�ط��ţ�����ʵ��ʽΪ____________��
��2����ͼ��A������ͼ��������Է�������Ϊ ________ ������ʽΪ _________��

��3�����ⶨ��A�ں˴Ź��������г����ĸ��壬���շ����֮��Ϊ6��1��2��1��A������Ʒ�Ӧ������������A�Ľṹ��ʽΪ ____________________________��
��4��A�ж���ͬ���칹�壬д����A������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ����д���֣���_________________________��_________________________��
���𰸡� C ��H ��O C4H10O 74 C4H10O ��CH3��2CHCH2OH CH3CH2CH2CH2OH CH3CH2��OH��CH2CH3����CH3��3C��OH��
����������1��3.7g�л���ȼ������0.2mol������̼��0.25 molˮ����n��C��=n��������̼��=0.2mol��m��C��=0.2mol��12g/mol=2.4g�� n��ˮ��=0.25mol��n��H��=0.5mol��m��H��=0.5mol��1g/mol=0.5g����m��C��+m��H��=2.4g+0.5g=2.9g��3.7g�����л��ﺬ��OԪ�أ���m��O��=3.7g-2.9g=0.8g����n��O��=0.05mol�� n��C����n��H����n��O��=0.2mol��0.5mol��0.05mol=4��10��1�������л������ʽΪC4H10O����ȷ�𰸣�C ��H ��O �� C4H10O��
��2��������ͼ��֪������Է�������Ϊ74��ʵ��ʽ��ʽ��Ϊ74����12��4+1��10+16=74���������ʽΪC4H10O����ȷ�𰸣�74��C4H10O��
��3�����л����ں˴Ź��������г����ĸ��壬����ԭ�Ӹ�����Ϊ6��1��2��1��˵���������⣬���л���������Ʒ�Ӧ����������˵�����ǻ�����A�Ľṹ��ʽΪ����CH3��2CHCH2OH����ȷ�𰸣���CH3��2CHCH2OH��
��4������һԪ��ͨʽΪCnH2n+2O�����Է���ʽΪC4H10O���л������Ϊ����һԪ���� ��A������ͬ������-�ǻ���ͬ���칹��Ľṹ��ʽ�ֱ�Ϊ��CH3CH2CH2CH2OH��CH3CH2��OH��CH2CH3����CH3��3C��OH����������ѡ���ּ��ɣ���ȷ�𰸣�CH3CH2CH2CH2OH��CH3CH2��OH��CH2CH3����CH3��3C��OH����������ѡ���ּ��ɣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�