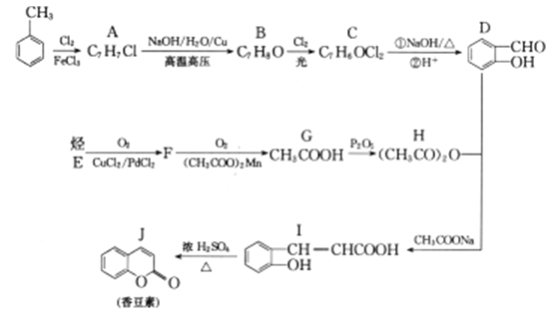
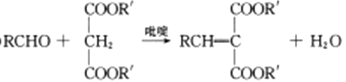
��Ŀ����
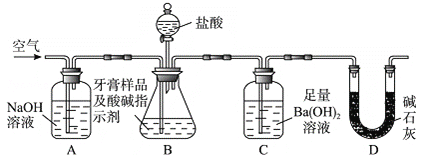
����Ŀ��������Ҫ�Ľ������ϣ�������(��Ҫ�ɷ���Al2O3��������SiO2��Fe2O3����)�ǹ�ҵ����ȡ����ԭ�ϡ�ʵ����ģ�ҵ����������Ϊԭ����ȡAl2(SO4)3�����������[NH4Al(SO4)2��12H2O]�Ĺ���������ͼ��ʾ��
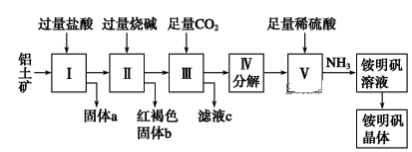
��ش��������⣺
��1������a�Ļ�ѧʽΪ________��
��2��д�����мӹ������ռ��漰���ķ�Ӧ���ӷ���ʽ______________��_____________������ͨ������CO2���巢����Ӧ�����ӷ���ʽΪ_______________��
��3���ɢ��еõ���Һc��ʵ�����Ϊ__________�����������Һ�л������������ʵ���������Ϊ(���������)________����ȴ�ᾧ������ϴ�ӡ�
��4����NH4Al(SO4)2��Һ��Ba(OH)2��Һ,�����ʵ�����1��2��ϣ�д�����ӷ���ʽΪ_______________��
��5����ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1��1����Ͷ��ʱ�������е�Al2O3��H2SO4�����ʵ���֮��Ϊ________��
���𰸡�SiO2 Al3+ + 4OH-= AlO2��+2H2O Fe3+ + 3OH- =Fe(OH)3�� AlO2����CO2��2H2O=HCO3����Al(OH)3�� ���� ����Ũ�� NH4+ +Al3+ + 2SO42-+2Ba2+ +4OH-=NH3H20 + Al(OH)3�� + 2BaSO4 �� 3��10
��������
�������������ܽ⣬Al2O3��Fe2O3��Ӧ�õ�AlCl3��FeCl3��SiO2�������ᷴӦ�����˵õ�����aΪSiO2����Һ�к���AlCl3��FeCl3��ʣ���HCl���ټ���������ռ��Ӧ�õ���������������ƫ�����ơ��Ȼ��ƣ����ˣ����ɫ����bΪ������������Һ�к���ƫ�����ơ��Ȼ��Ƽ�ʣ���NaOH��ͨ������Ķ�����̼����Ӧ������������������̼�����ƣ����ˣ���Һc�к����Ȼ��ơ�̼�����ƣ����������ȷֽ�õ����������������ܽ�õ���������Һ����ͨ�백�����õ��������Һ���������Ũ������ȴ�ᾧ�����˵Ȳ����õ���������壻����NH4Al(SO4)2��Һ��Ba(OH)2��Һ,�����ʵ�����1��2��Ϸ�Ӧ����������⣨4�������ӷ���ʽ����д�����Ƶõ�Al2(SO4)3��NH4Al(SO4)212H2O�����ʵ�������1mol������Al3+��SO42-�غ���㣬������⣨5�����
�������������ܽ⣬Al2O3��Fe2O3��Ӧ�õ�AlCl3��FeCl3��SiO2�������ᷴӦ�����˵õ�����aΪSiO2����Һ�к���AlCl3��FeCl3��ʣ���HCl���ټ���������ռ��Ӧ�õ���������������ƫ�����ơ��Ȼ��ƣ����ˣ����ɫ����bΪ������������Һ�к���ƫ�����ơ��Ȼ��Ƽ�ʣ���NaOH��ͨ������Ķ�����̼����Ӧ������������������̼�����ƣ����ˣ���Һc�к����Ȼ��ơ�̼�����ƣ����������ȷֽ�õ����������������ܽ�õ���������Һ����ͨ�백�����õ��������Һ���������Ũ������ȴ�ᾧ�����˵Ȳ����õ���������壻
��1��������Ϸ�����֪����������Al2O3��Fe2O3���������ᣬSiO2���������ᣬ���Թ���a�Ļ�ѧʽΪSiO2��
�����������������; SiO2��
��2��������Ϸ�����֪����������Al2O3��Fe2O3���������ᣬ�����Ȼ������Ȼ���������Һ�м���������ռAl3+��ΪAlO2����Fe3+��ΪFe(OH)3���������Ԣ��мӹ������ռӦ���ӷ���ʽΪ��Al3+ +4OH-= AlO2��+2H2O��Fe3+ +3OH- =Fe(OH)3����ƫ��������Һ��ͨ������CO2���巴Ӧ������������������̼�����ƣ����ӷ���ʽΪ��AlO2����CO2��2H2O=HCO3����Al(OH)3����
����������������ǣ�Al3+ +4OH-= AlO2��+2H2O��Fe3+ +3OH- =Fe(OH)3����AlO2����CO2��2H2O=HCO3����Al(OH)3����
��3��������Ϸ�����֪���ɢ��з�Ӧ��������������������̼��������Һ����˿��Բ��ù��˵ķ������з��룻����Һ�еõ�����IJ���һ��Ϊ����Ũ������ȴ�ᾧ������ϴ�ӣ����Դ��������Һ�л������������ʵ�������ȱ������Ũ����
����������������ǣ�����������Ũ����
��4����NH4Al(SO4)2��Һ��Ba(OH)2��Һ�������ʵ�����1��2��ϣ���Ӧ����һˮ�ϰ������ᱵ�����������������������ӷ���ʽΪNH4++Al3+ +2SO42-+2Ba2++4OH-=NH3H20 + Al(OH)3��+2BaSO4����
����������������ǣ�NH4+ +Al3+ +2SO42-+2Ba2+ +4OH-=NH3H20 +Al(OH)3��+2BaSO4����
��5�����Ƶõ�Al2(SO4)3��NH4Al(SO4)212H2O�����ʵ�������1mol����Al3+��3mol��SO42-��5mol������Al3+��SO42-�غ�ɵã�����Al2O3��H2SO4�����ʵ���֮��Ϊ3/2:5=3:10��
�������������������3��10��