��Ŀ����
����Ŀ��ij��Һ���ܺ���Na����K����Mg2����Cu2���������Ӽ�MnO![]() ��SiO
��SiO![]() ��AlO
��AlO![]() ��CO
��CO![]() ��HCO
��HCO![]() ��SO
��SO![]() ��Cl���������ӣ���֪���ٸ���Һ����ɫ���ھ��ⶨ��Һ��pH��12����ȡ������Һ����������100 mL 2 mol��L��1ϡ��������ữ���а�ɫ�������ɣ����õ�һ����ɫ��ζ�����壬������ʹ����ʯ��ˮ(����)����ǡ����ữ�����Һ���ˣ��õ���Һ�ס�
��Cl���������ӣ���֪���ٸ���Һ����ɫ���ھ��ⶨ��Һ��pH��12����ȡ������Һ����������100 mL 2 mol��L��1ϡ��������ữ���а�ɫ�������ɣ����õ�һ����ɫ��ζ�����壬������ʹ����ʯ��ˮ(����)����ǡ����ữ�����Һ���ˣ��õ���Һ�ס�
��1���ɢ٢ڢۿ��жϣ�ԭ��Һ��һ�������ڵ�������_____________��һ�����ڵ�������_____________��
��2������Һ�ֳ����ȷݣ�һ������μ��백ˮ�������а�ɫ��״������˵��ԭ��Һ��һ����_____________(�����ӷ���)���տ�ʼ���백ˮʱ��û�г���������ԭ����____________________(�����ӷ���ʽ��ʾ)����һ���м���������Ba(NO3)2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ��һ����_____________(�����ӷ���)�����˵õ���Һ�ҡ�
��3������Һ���м���������AgNO3��Һ�����ˡ�ϴ�ӡ�����ù���26.5 g����ԭ��Һ���Ƿ���Cl����_____________(��ǡ���)��
���𰸡�
��1��MnO![]() ��Cu2����Mg2����HCO
��Cu2����Mg2����HCO![]() ��CO
��CO![]() ��SiO
��SiO![]()
��2��AlO![]() ��NH3��H2O��H��=NH
��NH3��H2O��H��=NH![]() ��H2O��SO
��H2O��SO![]()
��3����
��������
�����������1���ٸ���Һ����ɫ������ɫ���Ӳ��ܴ��ڣ�һ��������MnO4-��Cu2+���ھ��ⶨ��Һ��pH=12���ڼ��Ի����²��ܹ�������Ӳ��ܴ��ڣ���������Mg2+��HCO3-����ȡ������Һ������ϡ����[100mL��2molL-1]�����ữ���а�ɫ�������ɣ����õ�һ����ɫ��ζ�����壬������ʹ����ʯ��ˮ(����)����ǣ���������Ƕ�����̼������ֻ���ǹ��ᣬһ������SiO32-��CO32-���ʴ�Ϊ��MnO4-��Mg2+��Cu2+��HCO3-��SiO32-��CO32-��
��2�����ữ�����Һ���ˣ��õ���Һ�ף�����һ������ʣ������ᣬ����Һ�ֳ����ȷݣ�һ������μ��백ˮ�������а�ɫ��״�������ܺͰ�ˮ��Ӧ�����İ�ɫ����ֻ����������������ʼʱ����ˮ�Ǻ����ᷢ���кͷ�Ӧ���кͷ�Ӧ�����ӷ�ӦΪNH3H2O+H+=NH4++H2O��������������������������һ���м���������Ba(NO3)2��Һ���а�ɫ����������ij������ɣ���һ�������ᱵ������֤��һ��������������ӣ��ʴ�Ϊ��AlO2-��NH3H2O+H+=NH4++H2O��SO42-��
��3��ȡ����ԭ��Һ������ϡ����[100mL��2molL-1��0.2mol]�����ữ���ֳ����ȷֺ�����ÿһ���к���0.1mol��HCl����Һ���м���������AgNO3��Һ�����ˡ�ϴ�ӡ�����ù���26.5g��n(AgCl)=![]() ��0.1mol������ԭ��Һ����Cl-���ʴ�Ϊ���ǡ�
��0.1mol������ԭ��Һ����Cl-���ʴ�Ϊ���ǡ�

 ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�����Ŀ��ij��ѧ��ȤС���ͬѧ�Ƕ�SO2��Ư�۾����������Ƶķ�Ӧ����ʵ��̽����
��ʵ��I��SO2��Ư�۾���Ӧ��
���� | ���� |
ȡ4g Ư�۾����壬����100mL ˮ | ���ֹ����ܽ⣬��Һ������ɫ |
���ˣ���Ư�۾���Һ��pH | pH ��ֽ�ȱ�����ԼΪ12��������ɫ |
| 1.Һ���Ϸ����ְ����� 2.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ�� 3.�Ժ���������ɫ����������ɫ��ȥ |
��1��Cl2��Ca(OH)2��ȡƯ�۾��ķ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__________��
��2��pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������____________��
��3����ȤС�齫A�в����İ����������������ữ��AgN03��Һ�У��г����������ݴ��жϰ����ɷ�ΪHClСҺ�Σ��ý��ۺ�����____________��˵������____________________��
��4������2����Һ��Ϊ����ɫ��ԭ���ǣ�����Һ���Ե���ǿ��Ư�۾�����Ч�ɷֺ�Cl-������Ӧ���÷�Ӧ�����ӷ���ʽΪ______________��
��5������Aƿ�л������ˡ�ϴ�ӣ��õ�����X,X�ijɷ���____________���ѧʽ����
�� �����ӷ���ʽ��������3�л���ɫ��ȥ��ԭ��____________________��
��ʵ��II��SO2��������Ʒ�Ӧ����һ������������SO2����ע����X�У�Ӳ�ʲ�����Y�м��������������ƣ������ü���K1��K2�кá��������°�ͼʾװ�ý���ʵ�飬����д�հס�
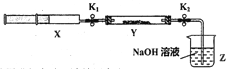
�������� | ʵ������ | ����ԭ�� |
��K1���ƶ�ע����������ʹX�е����建��ͨ��Y���У������ַ�Ӧ�� | ��1��_______ | ��2����Ӧ�Ļ�ѧ����ʽ��_________________ |
��ע���������˻�ԭ�����̶�����װ�ûָ������£���K2 | ��3��________ | ��4�����ý��� |