题目内容
已知苯甲酸乙酯的沸点为213℃(在此温度以下水、乙醇和环己烷以7.0%、17.0%、76.0%的比例成为蒸汽逸出)。请回答上述实验室制备苯甲酸乙酯的有关问题:
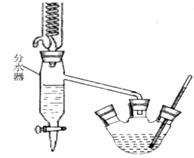
(1)①在三颈烧瓶中加入苯甲酸、浓硫酸、过量的乙醇、沸石;②再向该烧瓶中加入环己烷,装上分水器的回流冷却管。实验中使用分水器的目的是(从化学平衡原理分析)_______________。
(2)缓慢加热回流,至分水器下层液体不再增多,停止加热,放出分水器中液体,分水器中液体的三种主要成分是____________。
(3)将圆底烧瓶中的残液倒入盛有冷水的烧瓶中,用_____________溶液中和至弱碱性分液,分出粗产品;水层用乙醚____________(填实验操作名称),醚层与粗产品合并;用纯水洗有机层两次,将醚层与水尽量分净,醚层从上口倒入一个干燥的锥形瓶。
(4)加入适量豆粒大小的无水氯化钙干燥剂,摇动锥形瓶,至醚层澄清透明;醚层过滤入一个干燥的圆底烧瓶;进行____________(填实验操作名称),先蒸出__________后蒸出苯甲酸乙酯。

(1)①在三颈烧瓶中加入苯甲酸、浓硫酸、过量的乙醇、沸石;②再向该烧瓶中加入环己烷,装上分水器的回流冷却管。实验中使用分水器的目的是(从化学平衡原理分析)_______________。
(2)缓慢加热回流,至分水器下层液体不再增多,停止加热,放出分水器中液体,分水器中液体的三种主要成分是____________。
(3)将圆底烧瓶中的残液倒入盛有冷水的烧瓶中,用_____________溶液中和至弱碱性分液,分出粗产品;水层用乙醚____________(填实验操作名称),醚层与粗产品合并;用纯水洗有机层两次,将醚层与水尽量分净,醚层从上口倒入一个干燥的锥形瓶。
(4)加入适量豆粒大小的无水氯化钙干燥剂,摇动锥形瓶,至醚层澄清透明;醚层过滤入一个干燥的圆底烧瓶;进行____________(填实验操作名称),先蒸出__________后蒸出苯甲酸乙酯。
(1)分离反应过程中生成的水,促进酯化反应向正方向进行;(2)水、乙醇和环己烷;
(3)饱和碳酸钠溶液 萃取(4)蒸馏 乙醚
(3)饱和碳酸钠溶液 萃取(4)蒸馏 乙醚
试题分析:(1)苯甲酸和乙醇的酯化反应为可逆反应,水为生成物,实验中使用分水器的目的是分离反应过程中生成的水,促进酯化反应向正方向进行;(2)由题给信息水、乙醇和环己烷以7.0%、17.0%、76.0%的比例成为蒸汽逸出,分水器中液体的三种主要成分是水、乙醇和环己烷;(3)迁移教材乙酸乙酯的制备实验用饱和碳酸钠溶液来中和硫酸和苯甲酸,溶解乙醇,且苯甲酸乙酯在饱和碳酸钠溶液中的溶解度不大;由于苯甲酸乙酯易溶于乙醚,用乙醚来萃取水层中残余的苯甲酸乙酯;(4)乙醚与苯甲酸乙酯互溶且二者沸点不同,乙醚的沸点较低,故采用蒸馏的方法将二者分离,沸点低的乙醚先被分离出,后蒸出苯甲酸乙酯。

练习册系列答案
相关题目
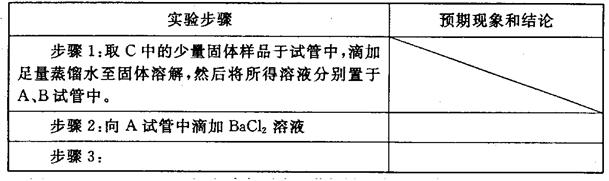

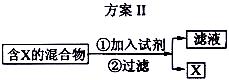
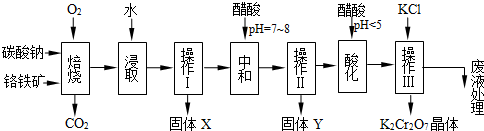
 8Na2CrO4 + 2 Fe2O3 + 8CO2↑;
8Na2CrO4 + 2 Fe2O3 + 8CO2↑; 2CrO42- + 2H+
2CrO42- + 2H+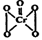 ), 该反应可用来检验Cr2O72-的存在。写出反应的离子方程式: 。
), 该反应可用来检验Cr2O72-的存在。写出反应的离子方程式: 。