��Ŀ����
�����ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3?H++H2PO3-��������������NaOH��Һ��Ӧ������Na2HPO3��
��1����д��������������NaOH��Һ��Ӧ�����ӷ���ʽ______��
��ij�¶��£�0.1000mol?L-1��H3PO3��ҺpH�Ķ���Ϊ1.6������ʱ��Һ��c��H+��=2.5��10-2mol?L-1����OH-֮���������ӵ�Ũ����С�����˳����______�����¶���H3PO3����ƽ���ƽ�ⳣ��K=______����H3PO3�ڶ���������Բ��ƣ����������λ��Ч���֣�
����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ��c��Na+��______c��H2PO3-��+2c��HPO32-�������������������=������
��2�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽ______��
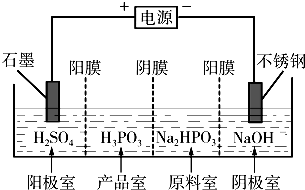
��3�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ��ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ��
�������ĵ缫��ӦʽΪ______��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ______��
��1����д��������������NaOH��Һ��Ӧ�����ӷ���ʽ______��
��ij�¶��£�0.1000mol?L-1��H3PO3��ҺpH�Ķ���Ϊ1.6������ʱ��Һ��c��H+��=2.5��10-2mol?L-1����OH-֮���������ӵ�Ũ����С�����˳����______�����¶���H3PO3����ƽ���ƽ�ⳣ��K=______����H3PO3�ڶ���������Բ��ƣ����������λ��Ч���֣�
����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ��c��Na+��______c��H2PO3-��+2c��HPO32-�������������������=������
��2�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽ______��
��3�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ��ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ��
�������ĵ缫��ӦʽΪ______��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ______��

��1�����������Ƕ�Ԫ�ᣬ������������������Ʒ�Ӧ����NaH2PO3��H2O�����Ը÷�Ӧ����ʽΪ��H3PO3+OH-=H2PO3-+H2O���ʴ�Ϊ��H3PO3+OH-=H2PO3-+H2O��
��0.1000mol?L-1��H3PO3��ҺpH�Ķ���Ϊ1.6��������Ũ��С��������Ũ�ȣ������������Ƕ�Ԫ���ᣬ��ˮ�зֲ����룬�ҵ�һ������̶ȴ��ڵڶ��������������ж������������ɣ�����������Ũ���������Ũ�ȴ�С˳����c��HPO32-����c��H2PO3-����c��H+����
H3PO3 ?H++H2PO3-
��ʼʱ������Ũ�ȣ�mol?L-1��0.1000
��Ӧ�ĸ����ʵ�Ũ�ȣ�mol?L-1��2.5��10-2 2.5��10-22.5��10-2
ƽ��ʱ�����ʵ�Ũ�ȣ�mol?L-1��0.10-2.5��10-2 2.5��10-22.5��10-2K=
=
=8.3��1
mol?L-1
�ʴ�Ϊ��c��HPO32-����c��H2PO3-����c��H+����8.3��10-3mol/L��
����Һ�����ԣ���C��H+��=C��OH-������Һ�ʵ����ԣ���c��Na+��+C��H+��=C��OH-��+c��H2PO3-��+2c��HPO32-������ΪC��H+��=C��OH-��������c��Na+��=c��H2PO3-��+2c��HPO32-�����ʴ�Ϊ��=��
��2�������ǿ�����ԣ����������ǿ��ԭ�ԣ�����������͵��ܷ���������ԭ��Ӧ�������������ᣬ��Ӧ����ʽΪ��
H3PO3+I2+H2O=2HI+H3PO4���ʴ�Ϊ��H3PO3+I2+H2O=2HI+H3PO4��
��3���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪ��HPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��
��0.1000mol?L-1��H3PO3��ҺpH�Ķ���Ϊ1.6��������Ũ��С��������Ũ�ȣ������������Ƕ�Ԫ���ᣬ��ˮ�зֲ����룬�ҵ�һ������̶ȴ��ڵڶ��������������ж������������ɣ�����������Ũ���������Ũ�ȴ�С˳����c��HPO32-����c��H2PO3-����c��H+����
H3PO3 ?H++H2PO3-
��ʼʱ������Ũ�ȣ�mol?L-1��0.1000
��Ӧ�ĸ����ʵ�Ũ�ȣ�mol?L-1��2.5��10-2 2.5��10-22.5��10-2
ƽ��ʱ�����ʵ�Ũ�ȣ�mol?L-1��0.10-2.5��10-2 2.5��10-22.5��10-2K=
| c(H+)?c(H2PO3-) |
| c(H3PO3) |
| 2.5��10-2��2.5��10-2 |
| 0.10-2.5��10-2 |
| 0 | -3 |
�ʴ�Ϊ��c��HPO32-����c��H2PO3-����c��H+����8.3��10-3mol/L��
����Һ�����ԣ���C��H+��=C��OH-������Һ�ʵ����ԣ���c��Na+��+C��H+��=C��OH-��+c��H2PO3-��+2c��HPO32-������ΪC��H+��=C��OH-��������c��Na+��=c��H2PO3-��+2c��HPO32-�����ʴ�Ϊ��=��
��2�������ǿ�����ԣ����������ǿ��ԭ�ԣ�����������͵��ܷ���������ԭ��Ӧ�������������ᣬ��Ӧ����ʽΪ��
H3PO3+I2+H2O=2HI+H3PO4���ʴ�Ϊ��H3PO3+I2+H2O=2HI+H3PO4��
��3���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪ��HPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��

��ϰ��ϵ�д�
�����Ŀ