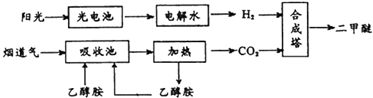
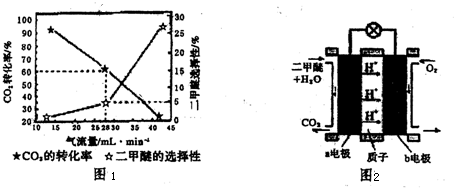
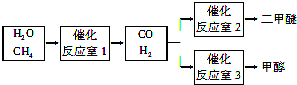
��Ŀ����
 ��1��������ȼ�ϵ�ص�������ӦʽΪ
��1��������ȼ�ϵ�ص�������ӦʽΪO2+4H++4e-�T2H2O
O2+4H++4e-�T2H2O
������ڷŵ�����У�������Χ��Һ��pH��С
��С
�������������С�����䡱����2�����������Ϊ��Դ��ͨ�����ߵ�������������Ϊʯī���������ҺΪ1L 0.1mol/L KCl��Һ��д������ܷ�Ӧ�����ӷ���ʽΪ
2Cl-+2H2O
H2��+Cl2��+2OH-
| ||
2Cl-+2H2O
H2��+Cl2��+2OH-
��
| ||
��3������ʱ����������2�����һ��ʱ���ȡ25mL����������Һ���μ�0.2mol/L ����õ���ͼ2��������������ʧ����������ˮ����Һ����仯���Բ��ƣ���
����ͼ2�е�B��pH=7����ζ��յ���
AB
AB
���䣨�AB������BC����CD������B����Һ������Ũ�ȴ�СΪc��CH3COO-��=c��K+����c��H+��=c��OH-��
c��CH3COO-��=c��K+����c��H+��=c��OH-��
����D����Һ��c��HAc��
��
��
c��Ac-�����������=������������1�����������ƶ������֪���缫Ϊ�������Ҳ�缫Ϊ������ȼ�ϵ���У������������õ��ӷ�����ԭ��Ӧ��������ȼ��ʧ���ӷ���������Ӧ�����ݸ�������������Ũ�ȱ仯�ж�pH�仯��
��2������Ȼ�����Һʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�л������������أ�
��3������Ϊ���ᣬǡ���к�ʱ��Һ�ʼ��ԣ�pH��7��C������������Һ�����ԣ�
��2������Ȼ�����Һʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�л������������أ�
��3������Ϊ���ᣬǡ���к�ʱ��Һ�ʼ��ԣ�pH��7��C������������Һ�����ԣ�
����⣺��1�����������ƶ������֪���缫Ϊ�������Ҳ�缫Ϊ������ȼ�ϵ���У������õ��Ӻ������ӷ�Ӧ����ˮ���缫��ӦʽΪO2+4H++4e-�T2H2O�������϶�����ʧ�������������ӣ����¸�������������Ũ��������Һ��pH��С��
�ʴ�Ϊ��O2+4H++4e-�T2H2O����С��
��2������Ȼ�����Һʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�л������������أ����Ե�ط�ӦʽΪ��2Cl-+2H2O
H2��+Cl2��+2OH-��
�ʴ�Ϊ��2Cl-+2H2O
H2��+Cl2��+2OH-��
��3���ٴ���Ϊ���ᣬǡ���к�ʱ��Һ�ʼ��ԣ�pH��7��ӦΪ��AB֮�䣬��Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ��c��CH3COO-��=c��K+ ��������Һ���������Ũ�ȴ���������Ũ�ȣ���������Ũ�ȴ�С˳����c��CH3COO-��=c��K+ ����c��H+��=c��OH-����
�ʴ�Ϊ��AB��c��CH3COO-��=c��K+ ����c��H+��=c��OH-����
�ڸ���ͼ��֪��������Һ��pH=13������������Ũ��Ϊ0.1mol/L��ȡ25mL����������Һ���μ�0.2mol/L���ᣬ��������Һ�����Ϊ25mLʱ����Һ�е������ǵ����ʵ����Ĵ����ƺʹ��ᣬD����Һ�����ԣ�˵����ĵ���̶ȴ���������ӵ�ˮ��̶ȣ�����c��HAc����c��Ac-����
�ʴ�Ϊ������
�ʴ�Ϊ��O2+4H++4e-�T2H2O����С��
��2������Ȼ�����Һʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�л������������أ����Ե�ط�ӦʽΪ��2Cl-+2H2O
| ||
�ʴ�Ϊ��2Cl-+2H2O
| ||
��3���ٴ���Ϊ���ᣬǡ���к�ʱ��Һ�ʼ��ԣ�pH��7��ӦΪ��AB֮�䣬��Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ��c��CH3COO-��=c��K+ ��������Һ���������Ũ�ȴ���������Ũ�ȣ���������Ũ�ȴ�С˳����c��CH3COO-��=c��K+ ����c��H+��=c��OH-����
�ʴ�Ϊ��AB��c��CH3COO-��=c��K+ ����c��H+��=c��OH-����
�ڸ���ͼ��֪��������Һ��pH=13������������Ũ��Ϊ0.1mol/L��ȡ25mL����������Һ���μ�0.2mol/L���ᣬ��������Һ�����Ϊ25mLʱ����Һ�е������ǵ����ʵ����Ĵ����ƺʹ��ᣬD����Һ�����ԣ�˵����ĵ���̶ȴ���������ӵ�ˮ��̶ȣ�����c��HAc����c��Ac-����
�ʴ�Ϊ������
���������⿼����ԭ��غ͵���ԭ��������Ũ�ȴ�С���жϣ����ݵ缫�ϵ�ʧ�����ж�ԭ������������������ӷŵ�˳��ȷ�������е�ط�Ӧʽ���ѵ���ͼ2�и������ʵ��жϣ��ѶȽϴ�

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
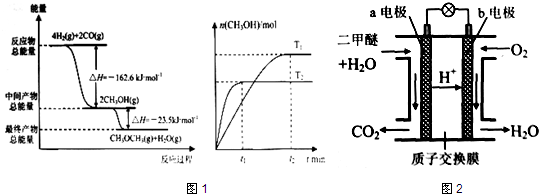
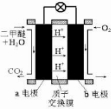 Ϊ��ɫ��Դ��ֱ�Ӷ�����ȼ�ϵ�ء�����ԭ��ʾ��ͼ��a�缫�ķ�ӦʽΪ
Ϊ��ɫ��Դ��ֱ�Ӷ�����ȼ�ϵ�ء�����ԭ��ʾ��ͼ��a�缫�ķ�ӦʽΪ