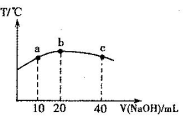
��Ŀ����
A��B��C��D��E����5��Ԫ�ء�����գ�
��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ�ط���Ϊ ��
��2��BԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�������ͬ��B��ԭ�ӽṹʾ��ͼΪ
��3��CԪ���ǵ�����������δ�ɶԵ��ӵ�����Ԫ�أ����Ĺ���Ų�ʽΪ ��
��4��DԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ��δ�ɶԵ��ӣ�D��̬ԭ�ӵĵ����Ų�ʽΪ
��5��Eԭ�ӹ���3���۵��ӣ�����һ���۵���λ�ڵ����ܲ�d�����ָ����Ԫ�������ڱ�����������������������
��1��N
��2��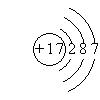
��3��1s2 2s2
2s2 2p6
2p6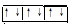 3s2
3s2
��4��[Ar]3d104s1
��5���� ��B
���������������1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ�˵����Ԫ����2����ӣ������5�����ӣ���Ԫ��Ϊ7��Ԫ��NԪ��
��2��BԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�������ͬ��˵��BԪ��Ϊ17��Ԫ����Ԫ�أ���ԭ�ӽṹʾ��ͼ
��3��CԪ���ǵ�����������δ�ɶԵ��ӵ�����Ԫ�أ���CΪþԪ�أ����Ĺ���Ų�ʽ1s2 2s2
2s2 2p6
2p6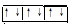 3s2
3s2
(4)DԪ�ص�M��ȫ������N��ֻ��1��������DΪ29��Ԫ�أ���̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1
(5)Eԭ�ӹ���3���۵��ӣ�����һ���۵���λ�ڵ����ܲ�d�����˵������2���۵���λ�ڵ����ܲ�s���������E�ļ۵����Ų�Ϊ3d14s2��λ�����ڱ��е������ڵ�������
���㣺����ԭ�ӽṹ��Ԫ�������ڱ��е�λ�ù�ϵ��ԭ�ӽṹʾ��ͼ�������Ų�ʽ�������Ų�ͼ������

���Ϊ�١����Ԫ�أ���Ԫ�����ڱ��е�λ�����£�
| ���� ���ڡ� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 1 | �� | | | | | | | �� |
| 2 | | | | �� | �� | �� | �� | |
| 3 | �� | �� | | | | �� | �� | |
�Իش��������⣺
(1)��ԭ��ֻҪ�γ�һ�Թ��õ��ӶԾʹﵽ���ȶ��ṹ��Ԫ����________��(��дԪ�ط���)
(2)�ٺܺ͢�Ԫ���γɵĻ�����Ļ�ѧʽΪ________���õ���ʽ��ʾ���γɹ���Ϊ________��
(3)���Ԫ�ص�����������ˮ����Ļ�ѧʽ��____________________��
(4)�١��ݡ��ߺ�Ԫ���γɵ�һ�ֻ�����ĵ���ʽ��________���ڸû������мȺ���________�����ֺ���________����

 ��
�� ���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ�������________(ѡ����ţ���ͬ)��
���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ�������________(ѡ����ţ���ͬ)��