��Ŀ����
��ҵ�ϣ�����ͭ��CuFeS2��ͨ��8CuFeS2+21O2 8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
��1��������Ӧ�У���ԭ��Ϊ ��
��2����ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
��������Ϣ�ش��������⣺
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
��3��������¯���н��к������IJⶨ�������£�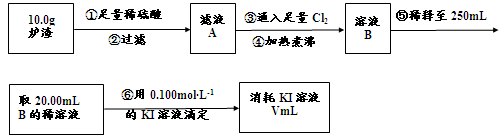
I������۷�����Ӧ�����ӷ���ʽΪ ��
II����������������� ��
III����������õ��IJ����������ձ�������������ͷ�ιܡ� ��
IV�����ζ�����������0.100mol��L?1KI��Һ20.00mL����¯�������İٷֺ���Ϊ ��
��1��CuFeS2 ��2��a��[Al(OH)4]-��AlO2- b��ϡ���ᡢ����KMnO4��Һ��ϡ�����ȡ¯��������Һʹ����KMnO4��Һ��ɫ��3��I��2Fe2++Cl2= 2Fe3++2Cl-II��������Һ���ܽ�Ĺ�����Cl2 III��250mL����ƿ ��δ��250mL�������֣�IV��14%
���������������1���ڷ�Ӧ��CuFeS2����Ԫ�صĻ��ϼ���+2�۱�Ϊ+3�ۣ���Ԫ�صĻ��ϼ���-2�۱�Ϊ+4������ԭ������2������ϡ�����ȡ¯�������ķ�ӦΪ��Al2O3 + 6H+=2Al3+ + 3H2O
FeO + 2H+ = Fe2+ + H2O Fe2O3 + 6H+ = 2Fe3+ + 3H2O ����Һ�к��еĽ�����������Al3+��Fe2+��Fe3+����������Al3+��Fe3+���ӹ�������������Һ�����ķ�Ӧ��Al3+ + 4OH-=AlO2- + 2H2O Fe3+ + 3OH- = Fe(OH)3�� a��ͨ�������ڣ�¯���е�Al2O3�����AlO2-��b��֤¯���к���+2�۵�����Ӧ�ȼ���ϡ�����ܽ⣬�����������ӣ����л�ԭ�ԣ����������ط���������ԭ��Ӧ���ữʱ���ܼ������ᣬ�����������ط���������ԭ��Ӧ��Ҳ����ֱ�Ӽ������ᣬ�������������ӣ����ܼ��飬�ʴ�Ϊ��ϡ���ᡢKMnO4��Һ��ϡ�����ȡ¯��������Һ�����������ӣ��������ط���������ԭ��Ӧ��ʹ���������ɫ���ʴ�Ϊ��ϡ�����ȡ¯��������Һ��ʹKMnO4��Һ��ɫ����3��������������ͼ�����ķ�ӦΪ��¯��������ϡ���Al2O3 + 6H+=2Al3+ + 3H2O FeO + 2H+ = Fe2+ + H2O Fe2O3 + 6H+ = 2Fe3+ + 3H2O���ˣ���Һ�к��еĽ�����������Al3+��Fe2+��Fe3+��ͨ������Cl2��2Fe2+ + Cl2 = 2Fe3+ + 2Cl- �ζ�ԭ����2Fe3+ + 2I- = 2Fe2+ + I2I�������ͨ����������������Ӧ�����ӷ���ʽΪ2Fe2+ + Cl2 = 2Fe3+ + 2Cl- ��II������������������I-����Ӱ��ʵ���������Բ��������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2����III�������Ϊ��Һ�����ƣ��õ��IJ����������ձ�������������ͷ�ιܡ�250mL����ƿ��IV���ζ������з����ķ�ӦΪ2Fe3+ + 2I- = 2Fe2+ + I2���ɷ�Ӧʽ֪n(I-)= n(Fe3+)=0.002mol���������ͼ֪10.0g¯�����������ʵ���Ϊ0.025mol������Ϊ1.4g,��������Ϊ14%��
���㣺�Ի�ѧ��������Ϊ���忼��������ԭ��Ӧ��������������Ļ���������ʡ���Һ�������Լ���ϵʽ�����㡣

��Ԫ�ؼ��仯�������������������ϢϢ��أ��Իش��������⣺
��1�����ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�壬�÷�Ӧ�����ӷ���ʽΪ ��
��2����֪��Fe(s)+ O2(g)
O2(g)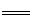 FeO(s) ��H=��272 kJ��mol��1
FeO(s) ��H=��272 kJ��mol��1
C(s)+O2(g) CO2(g) ��H=��393.5 kJ��mol��1
CO2(g) ��H=��393.5 kJ��mol��1
2C(s)+O2(g) 2CO(g) ��H=��221 kJ��mol��1
2CO(g) ��H=��221 kJ��mol��1
���¯���������� FeO(s)+CO(g) Fe(s)+CO2(g) ��H= ��
Fe(s)+CO2(g) ��H= ��
��3�����죨Fe2O3����һ�ֺ�ɫ���ϡ���һ��������������160mL 5 mol��L��1�����У��ټ����������ۣ�����Ӧ�������ռ�������2.24L����״�������������Һ����Fe3������μӷ�Ӧ�����۵�����Ϊ ��
��4����H2��O2��������Na2CO3���ȼ�ϵ�أ����õ�ⷨ�Ʊ�Fe(OH)2��װ������ͼ��ʾ������P��ͨ��CO2��
��ʯīI�缫�ϵĵ缫��ӦʽΪ ��
��ͨ��һ��ʱ����Ҳಣ�����в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ��������˵������ȷ���� ������ţ���
| A��X��Y���˶������������缫 |
| B��������NaOH��Һ��Ϊ���Һ |
| C�����������ķ�Ӧ�ǣ�2H2O�� 2e��= H2��+ 2OH�� |
| D����ɫ����ֻ���������ϲ��� |
��1��Ԫ��M�Ƕ�����Ԫ�أ��䳣�������ں�ˮ�У����ʱ���Ϊ��������������
��M��ԭ�ӽṹʾ��ͼΪ______��
����M��AlΪ�缫��KOH��ҺΪ�������Һ�����ĵ缫��ӦʽΪ______��
��2������ǽ������������ȵ�ij�¶ȣ��漴�����������п�����ȴ�Ľ����ȴ������ա�
��ʹ��ˮ���д�������ɴ������������÷�Ӧ�Ļ�ѧ����ʽΪ____________
����֤����ˮ����Ĺ�������Ƿ����+3�۵�������ѡ�õ��Լ�Ϊ_______ (�����)
| A��H2O2��Һ | B��ͭ�� | C��ϡ���� | D��KMnO4��Һ |
 4Fe(OH)3+8OH��+3O2
4Fe(OH)3+8OH��+3O2��ͼ1��25��ʱK2FeO4�ڲ�ͬpH��Һ��Ũ�ȵı仯�����pH =4.74ʱ����Ӧ�ӿ�ʼ��800min��ƽ����Ӧ����v(FeO42��)=______ (������λ��Ч���֣���
��ͼ1��800min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䡣�۲�ͼ1�ж�����pH ��˷�Ӧ��ƽ�ⳣ��______����������С�����䡱����
��ͼ2��240min��������Һ��K2FeO4��Ũ�Ⱦ����ٸı䣬��������Ӧ�ķ�Ӧ�ȡ�H______0(�>������<������=������

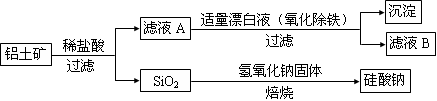
����ʯ����������������þ����Ҫ�����������£�
I����ȡ������þ������Һ��������ʯ��ۣ����ˣ����ڳ��³�ѹ�½ᾧ���Ƶô�����þ�����г���������Fe3+��Al3+��Fe2+���������ӣ���
II���ᴿ������þ����������þ�������������ܽ⣬����������0.1 mol��L��H2O2��Һ���ٵ�����ҺpH��7��8���������ᴿ��
III����ȡ������þ������II������Һ�м��������ˮ��
��֪�������������������������pH
| | Fe3+ | Al3+ | Fe2+ | Mg2+ |
| ��ʼ����ʱ | 1.5 | 3.3 | 6.5 | 9.4 |
| ������ȫʱ | 3.7 | 5.2 | 9.7 | 12.4 |
��ش��������⣺
��1������II�У������ڵ�����ҺpH��7~8������Լ��� ������ĸ��ţ���
A. MgO B. Na2CO3 C. ����ˮ
��2��Fe2+��H2O2��Һ��Ӧ�����ӷ���ʽΪ ��
��3����ҵ�ϳ���Mg2+��ת����Ϊ����ָ�꣬ȷ������III�Ʊ�������þ���չ��̵��������������У���Ӧ�¶���Mg2+ת���ʵĹ�ϵ����ͼ��ʾ��
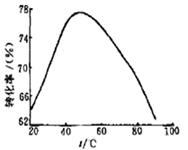
�ٲ���III���Ʊ�������þ��Ӧ�����ӷ���ʽΪ ��
�ڸ���ͼ����ʾ50 ��ǰ�¶���Mg2+ת����֮�� �Ĺ�ϵ�����жϴ˷�Ӧ��
������ȡ����ȡ�����Ӧ��
��ͼ�У��¶�������50 ������Mg2+ת�����½��Ŀ���ԭ���� ��
�� Ksp��ʾ�����ܽ�ƽ���ƽ�ⳣ������֪��
Mg(OH)2(s)
 Mg2+ (aq)+ 2OH- (aq) Ksp = c(Mg2+)��c2(OH-) = 5.6��10-12
Mg2+ (aq)+ 2OH- (aq) Ksp = c(Mg2+)��c2(OH-) = 5.6��10-12Ca(OH)2(s)
 Ca2+ (aq) + 2OH- (aq) ��sp = c(Ca2+)��c2(OH-) = 4.7��10-6
Ca2+ (aq) + 2OH- (aq) ��sp = c(Ca2+)��c2(OH-) = 4.7��10-6����ʯ���������ˮ�� ����ܡ����ܡ����Ƶ�������þ�������� ��
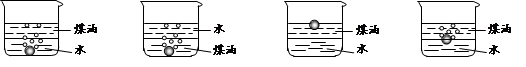
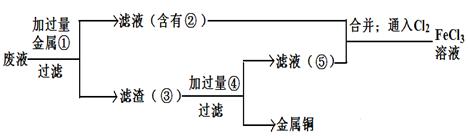
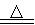 AlCl3��X�������ʵ��ȷ������X�ijɷ֣�
AlCl3��X�������ʵ��ȷ������X�ijɷ֣�