��Ŀ����
����Ŀ�����������仯��������Ҫ�Ͻ���Ϻʹ������䴢��Ͻ����Ϊһ������п���ӵ�صĸ������ϣ��õ����Zn(CF3SO3)2Ϊ����ʣ�����ȱ�ݵ���������ZnMn2O4Ϊ�缫���ɹ�������ȶ��Ĵ��ʵ�����
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ________�����Ų�ʱ������ߵ�����ռ���ܼ���ԭ�ӹ����________����չ����
��2��VO2+������������γ���������ͬ�����ҵ�һ�����ܱ����������Ԫ����____(дԪ�ط���)��
��3�����γɵ�������[Ni��NH3��6]2+��[Ni(CN)4]2-�У�NH3���ӵĿռ乹��Ϊ_______����CN-��Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽΪ__________��
��4����������(CF3SO3H)��һ���л�ǿ�ᣬ�ṹʽ��ͼ1��ʾ��ͨ����CS2��IF3��H2O2��Ϊ��Ҫԭ������ȡ��
��H2O2������Oԭ�ӵ��ӻ���ʽΪ________��
��������������ⱽ��Ӧ�����������ᱽ���͵⻯�⡣1���������ᱽ�������к�����������ĿΪ__________��

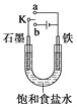
��5����п����Ĺ����ж��֣�����һ����п�ľ�����ͼ2��ʾ���þ�����S2-����λ��Ϊ____��
��6�������Ͻ�����Ҫ������ϣ��䴢���ľ�����ͼ3��ʾ��
�ٴ���ǰ�������Ͻ�Ļ�ѧʽΪ___________��
�ڸ������Ͻ�����������ܶ�Ϊ________(��NA��ʾ�����ӵ���������ֵ)g.cm-3��
���𰸡� [Ar]3d34s2 5 N��F ������ N2��CO sp3 19 4 LaNi5 ![]()
����������1����̬��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d34s2��[Ar]3d34s2�����Ų�ʱ������ߵĵ�����ռ���ܼ�Ϊ3d��3dԭ�ӹ����5����չ������ȷ�𰸣�[Ar]3d34s2��5��
��2����ԭ���ڵڶ�����VIA�壬ͬһ���ڴ������ң�����ͬ�����ҵ�һ�����ܱ����������Ԫ���е�ԭ�ӡ���ԭ�ӣ���ȷ�𰸣�N��F��
��3�����������е�ԭ�Ӽ۵��Ӷ���ĿΪ3+1/2����5-1��3��=4�����Բ���sp3���ռ乹��Ϊ�����Σ���CN-��Ϊ�ȵ����壨������14����һ�ַ��ӵĻ�ѧʽΪN2��CO����ȷ�𰸣���������N2��CO��
��4����H2O2����H-O-O-H��Oԭ�ӳ�2���Ҽ�����2�Թ¶Ե��ӣ�ԭ���ӻ������=�ļ���+�¶Ե��Ӷ���=2+2=4��������sp3�ӻ�����ȷ�𰸣�sp3��
�ڸ��ݸ��л���Ľṹ������1�������к��ЦҼ���3�� C-F ����1��C-S ����2�� S=O ���� 1�� S-O����1��C-0���������Ϻ���5��C-H����6�� C-C ��������1���������ᱽ�������к��ЦҼ�����ĿΪ19����ȷ�𰸣�19��
��5�����ݾ����ṹͼ�������к���S2-��Zn2+����λ����ȣ���������Zn2+����Ҿ�����ȵ�S2-��4������λ��Ϊ4����ȷ�𰸣�4��
��6���ٸ��ݾ����ṹͼ������ǰ�������к�����ԭ����=8��1/8=1, ��ԭ����8��1/2+1=5���Ͻ�Ļ�ѧʽΪLaNi5����ȷ�𰸣�LaNi5��
�ھ���������������Ŀ8��1/4+2��1/2=3.�����ĺϽ�ѧʽΪLaNi5H6,�����1mol���������Ϊ��(a��10-10)3��NA= a3��10-30 ��NAcm3,1mol�����к��е���������Ϊ6g���������Ͻ������ܶ�Ϊ��6����a3��10-30 NA��g/cm3����ȷ�𰸣�6����a3��10-30 NA����

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�����Ŀ����(Co)���仯�����ڹ�ҵ���й㷺Ӧ�á�����ˮ�ܿ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��A12O3��MnO��MgO��CaO�ȣ���ȡCoC12��6H2O��Ʒ�Ĺ�������ͼ���£�

��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��A13+�ȣ�
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��pH���±���
������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��ش��������⣺
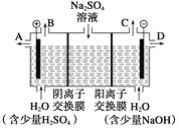
(1)������������ͼ�в���a�����ƣ�_______________��
(2)д����������ʱCo2O3������Ӧ�����ӷ���ʽ��____________________��
(3)����NaC1O3��������________________ ��
(4)��ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ��ʹ����ȡ�������˵�pH��Χ��_________(����ĸ���)��
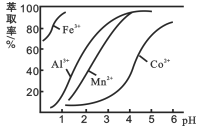
A.2.0��2.5 B. 3.0��3.5 C. 5.0��5.5 D. 9.5��9.8
(5)�����ơ���þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪ij�¶��£�Ksp(MgF2)=7.35��10-11�� Ksp(CaF2)=1.50��10-10�����������NaF��������Һ��c(Mg2+)/c(Ca2+)=_______________��