��Ŀ����
����Ŀ����Ԫ���ܹ��γɶ��ֻ������ش��������⣺
![]() ����
����![]() ������ΪҺ̬���ڿ�����Ѹ����ȫȼ������
������ΪҺ̬���ڿ�����Ѹ����ȫȼ������![]() ��ͬʱ�ų������ȣ���������������ɴ��������ȼ�ϡ�
��ͬʱ�ų������ȣ���������������ɴ��������ȼ�ϡ�
��֪��![]() ��ȼ����Ϊ
��ȼ����Ϊ![]()
![]() ��
��![]()
![]() ��
��![]()
��![]() �ڿ�����ȼ��������̬ˮ���Ȼ�ѧ����ʽΪ______��
�ڿ�����ȼ��������̬ˮ���Ȼ�ѧ����ʽΪ______��
![]() ��ҵ�����ð�������������
��ҵ�����ð�������������![]() �ķ�ӦΪ
�ķ�ӦΪ![]()
![]() ��
��
![]() һ���¶��£���2L���������г���1mol
һ���¶��£���2L���������г���1mol![]() ��2mol
��2mol![]() ����������Ӧ��8min�ﵽƽ��ʱ�����
����������Ӧ��8min�ﵽƽ��ʱ�����![]() ��ת����Ϊ
��ת����Ϊ![]() ��
��![]() �ڣ���
�ڣ���![]() ��ʾ�ĸ÷�Ӧ����v
��ʾ�ĸ÷�Ӧ����v![]() ______��
______��
�����¶Ⱥ��ݻ����䣬����ƽ���������г���![]()
![]() ��
��![]() HCN����ʱ
HCN����ʱ![]() ______
______![]() ѡ����
ѡ����![]() ����
����![]() ������
������![]() ��
��![]() ��
��
![]() ��
��![]() �£���a
�£���a![]() ��NaCN��Һ��
��NaCN��Һ��![]() ������������ϣ���Ӧ������Һ
������������ϣ���Ӧ������Һ![]() ����a______
����a______![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ���ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��
���ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��![]() ______��
______��
![]() �ܹ���
�ܹ���![]() �γ�
�γ�![]() ��
��
![]() ��Һ�д���
��Һ�д���![]()
![]()
![]()
![]()
![]() ʱ����ƽ�ⳣ���ı���ʽΪ
ʱ����ƽ�ⳣ���ı���ʽΪ![]() ______��
______��
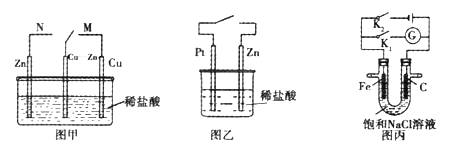
![]() ���������������кܴ��ԣ��绯ѧ����
���������������кܴ��ԣ��绯ѧ����![]() ��ԭ����ͼ��
��ԭ����ͼ��
![]() ������ӦʽΪ______��
������ӦʽΪ______��
![]() ����������ת����3mol���ӣ���Ĥ������Һ�������仯��
����������ת����3mol���ӣ���Ĥ������Һ�������仯��![]() Ϊ______g��
Ϊ______g��
���𰸡�![]()
![]()
![]()
![]()
![]()
![]()
![]() 2NO2-+6e-+8H+=N2
2NO2-+6e-+8H+=N2![]() +4H2O 16
+4H2O 16
��������
![]() д��������ȼ���ȵ��Ȼ�ѧ����ʽ��Ȼ����ݸ�˹���ɼ��ɽ��
д��������ȼ���ȵ��Ȼ�ѧ����ʽ��Ȼ����ݸ�˹���ɼ��ɽ��
![]() ���ݷ�Ӧ����
���ݷ�Ӧ����![]() ���ɼ���������Ĺ�ϵ���ҳ����ĵ������������ٸ��ݹ�ʽ����¶Ȳ��䣬ƽ�ⳣ���Ͳ��䣬Ȼ���������Ũ�Ȼ���ƽ�ⳣ���Ĵ�С�Ƚϣ����ж�ƽ�����ƶ����Ӷ��ó�ʱ
���ɼ���������Ĺ�ϵ���ҳ����ĵ������������ٸ��ݹ�ʽ����¶Ȳ��䣬ƽ�ⳣ���Ͳ��䣬Ȼ���������Ũ�Ȼ���ƽ�ⳣ���Ĵ�С�Ƚϣ����ж�ƽ�����ƶ����Ӷ��ó�ʱ![]() ��
��![]() �Ĺ�ϵ����Һ�����������Һ�������������������ӵ���Դ�С���ٶ�
�Ĺ�ϵ����Һ�����������Һ�������������������ӵ���Դ�С���ٶ�![]() �������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ������
�������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ������![]() ʱ����
ʱ����![]() ���õ���غ�������غ����ҳ���Һ�е����Ӵ�С��ϵ��Ȼ���ڽ����볣������������ɣ�
���õ���غ�������غ����ҳ���Һ�е����Ӵ�С��ϵ��Ȼ���ڽ����볣������������ɣ�
![]() ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮����Ȼ���ҳ�k��Ksp�Ĺ�ϵʽ������ã�
ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮����Ȼ���ҳ�k��Ksp�Ĺ�ϵʽ������ã�
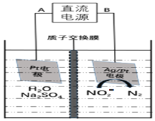
![]() ���Ĺ���ԭ����NO2-�������õ����ӣ�д���缫����ʽ��2NO2-+6e-+8H+=N2
���Ĺ���ԭ����NO2-�������õ����ӣ�д���缫����ʽ��2NO2-+6e-+8H+=N2![]() +4H2O���������������ʵı仯���ҳ����ʵ�ȥ������������IJ��
+4H2O���������������ʵı仯���ҳ����ʵ�ȥ������������IJ��
![]() �������֪��������ȼ����Ϊ
�������֪��������ȼ����Ϊ![]() ����
����![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
���ݸ�˹���ɣ�![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() �ﵽƽ��ʱ�����ļ�������ʵ���
�ﵽƽ��ʱ�����ļ�������ʵ���![]() ���ɷ�Ӧ����ʽ��֪������
���ɷ�Ӧ����ʽ��֪������![]() �����ʵ���Ϊ�����3������
�����ʵ���Ϊ�����3������![]() ��0��8min�ȣ���������ʾ�ĸ÷�Ӧ����
��0��8min�ȣ���������ʾ�ĸ÷�Ӧ����![]() =
=![]() ,���ݷ�Ӧ����ʽ��������������֪���ﵽƽ��ʱ��
,���ݷ�Ӧ����ʽ��������������֪���ﵽƽ��ʱ��![]() ��
��![]() ��
��![]()
![]() ��
��![]() ����ƽ�ⳣ��
����ƽ�ⳣ��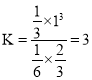 ������ƽ���������г�
������ƽ���������г�![]()
![]() ��
��![]() HCN��
HCN��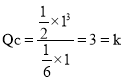 ��ƽ�ⲻ�ƶ���
��ƽ�ⲻ�ƶ���![]() ������������ȣ��ʴ�Ϊ��=��
������������ȣ��ʴ�Ϊ��=��
![]() ����
����![]() �������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ����
�������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ����![]() ʱ��
ʱ��![]() �������ϣ�����Ũ�Ⱦ����롣��Ӧ����Һ
�������ϣ�����Ũ�Ⱦ����롣��Ӧ����Һ![]() ��
��![]() �����ݵ���غ�ã�
�����ݵ���غ�ã�![]() ����
����![]() ��
��![]() �����������غ�ã�
�����������غ�ã�![]() ����
����![]() ��HCN�ĵ��볣��
��HCN�ĵ��볣��![]()
�ʴ�Ϊ��![]()
![]() ��
��
![]() ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮������ƽ�ⳣ���ı���ʽ
ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮������ƽ�ⳣ���ı���ʽ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ��װ��ͼ����Ϣ��֪�缫BΪ�����������õ����ӣ����缫��ӦʽΪ2NO2-+6e-+8H+=N2
��װ��ͼ����Ϣ��֪�缫BΪ�����������õ����ӣ����缫��ӦʽΪ2NO2-+6e-+8H+=N2![]() +4H2O���ʴ�Ϊ��2NO2-+6e-+8H+=N2
+4H2O���ʴ�Ϊ��2NO2-+6e-+8H+=N2![]() +4H2O��
+4H2O��
����ͼʾ��֪������Ϊ![]() ������3mol����ͨ��ʱ����������
������3mol����ͨ��ʱ����������![]() ��������3mol�����ӽ��������ң���������������27g��������
��������3mol�����ӽ��������ң���������������27g��������![]() ��֪������
��֪������![]() ��N2����14g��ͬʱ��
��N2����14g��ͬʱ��![]() ��3g���룬�������Ҽ�������Ϊ11g����Ĥ������Һ�������仯��
��3g���룬�������Ҽ�������Ϊ11g����Ĥ������Һ�������仯��![]() ���ʴ�Ϊ��16��
���ʴ�Ϊ��16��