��Ŀ����
����Ŀ����l����Ȼ����CO2ͨ��۽�̫���ܷ�Ӧ����������ӦCH4(g)+ CO2(g)![]() 2CO(g)+2H2(g) ��H����֪��CH4��H2��CO��ȼ������H�ֱ�Ϊ��akJ/mol����bkJ/mol����ckJ/mol����������Ӧ�У���H��_______________���ú�a��b��c�Ĵ���ʽ��ʾ��kJ/mol��
2CO(g)+2H2(g) ��H����֪��CH4��H2��CO��ȼ������H�ֱ�Ϊ��akJ/mol����bkJ/mol����ckJ/mol����������Ӧ�У���H��_______________���ú�a��b��c�Ĵ���ʽ��ʾ��kJ/mol��
��2��CO2��H2���Ժϳɶ����ѣ�CH3OCH3������������һ����ɫ��������Դ����ϡ����Ϊ�������Һ����ij�����ѿ���ȼ�ϵ��ÿ����l mol CH3OCH3����·��ͨ��9 mol���ӣ���õ��Ч����Ϊ___________������ʾ�����Ч�ʵ��ڵ�·��ͨ���ĵ��������ط�Ӧ��ת�Ƶ�������֮�ȣ�
��3��325Kʱ���ں����ܱ������г���һ����NO2 ,������Ӧ��2NO2(g)![]() N2 O4(g)��
N2 O4(g)��
��NO2��N2O4��������������Ũ�ȵĹ�ϵ��ͼ��ʾ��ͼ�н���A��ʾ�÷�Ӧ������״̬Ϊ��____________��
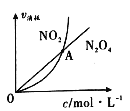
A��ƽ��״̬ B��������Ӧ�����ƶ� C�����淴Ӧ�����ƶ� D�����ж�
�� �ܹ�˵��������Ӧ�Ѿ��ﵽƽ��״̬����__________________��
a��NO2��N2 O4�����ʵ������ b����ϵ��ɫ���ٱ仯
c�������ڻ��������ܶȲ��ٸı� d������������ѹǿ�������仯
�����ﵽƽ��������ڻ�������ƽ����Է�������Ϊ57.5��ƽ��ʱNO2��ת����Ϊ_____��
��4��ʵ���ж����SO2����������������Һ���ա�������H2SO3��Ka1��1.3��10��2��Ka2��6.6��10��8��
��������pH=3����������Һ�������ж���ȷ����_______________��
a����Һ��c(H2SO3)��c(SO32��) b����Һ��c(SO32��)��c(HSO3��)��6.6��10��5
c����Һ����������ĿΪ6.02��1020�� d����������pH��3��������Һ��������ĵ���ƽ�ⲻ�ƶ�
�ڳ����£��ú�1mol�������Ƶ�ϡ��Һ��������SO2���壬�ų�����ΪQkJ���÷�Ӧ���Ȼ�ѧ����ʽΪ_______
��������Һ��c(HSO3��)��c(SO32��)������Һ��c(H+)��________________��
���𰸡���2c+2b��a��75%Cbd40%a b dNaOH(aq)��SO2(g)��NaHSO3(aq) ��H����QkJ/mol6.6��10-8 mol��L��1
��������
(l) ��֪����CH4(g)+2O2=CO2(g)+2H2O(l)��H=-akJmol-1����H2(g)+![]() O2(g)=H2O(l)��H=-bkJmol-1����CO(g)+
O2(g)=H2O(l)��H=-bkJmol-1����CO(g)+ ![]() O2(g)=CO2(g)��H=-ckJmol-1���ɸ�˹���ɿ�֪����-����2-����2��֪CH4(g)+ CO2(g)
O2(g)=CO2(g)��H=-ckJmol-1���ɸ�˹���ɿ�֪����-����2-����2��֪CH4(g)+ CO2(g)![]() 2CO(g)+2H2(g) ��H=+(2c+2b��a)kJmol-1���ʴ�Ϊ��+(2c+2b��a)��
2CO(g)+2H2(g) ��H=+(2c+2b��a)kJmol-1���ʴ�Ϊ��+(2c+2b��a)��
(2) l mol CH3OCH3��������Ӧ���ɶ�����̼��ˮ��ת�Ƶ���12mol����·��ͨ��9 mol���ӣ���õ��Ч����=![]() ��100%=75%���ʴ�Ϊ��75%��
��100%=75%���ʴ�Ϊ��75%��
(3) ����ﵽƽ��ʱ�����ĵ�����2v(N2O4)=v(NO2)��������A��ʾ���ĵ�����v(N2O4)=v(NO2)���ɴ˿�ȷ����ʱNO2����������С�������������� N2O4����ƽ�������ƶ����ʴ�Ϊ��C��
��a����ƽ�ⳣ��δ֪����NO2��N2O4�����ʵ�����Ȳ�һ���ﵽƽ��״̬����a����b����ϵ��ɫ���ڱ仯����˵��Ũ�Ȳ��ٸı䣬�ﵽƽ��״̬����b��ȷ��c����������������䣬������������䣬�������Ƿ�ﵽƽ��״̬�������ڻ��������ܶȶ����ı䣬��c����d����Ӧǰ�������������ȣ�����������ѹǿ�������仯����˵���ﵽƽ��״̬����d��ȷ���ʴ�Ϊ��bd��
��4.6g NO2�����ʵ���Ϊ0.1mol����ƽ��ʱת��xmol����
2NO2(g) N2O4(g)
��ʼ(mol)��0.1 0
ת��(mol)�� x 0.5x
ƽ��(mol)��0.1-x 0.5x
�ﵽƽ��������ڻ�������ƽ����Է�������Ϊ57.5����![]() =57.5�����x=0.04��ת����Ϊ
=57.5�����x=0.04��ת����Ϊ![]() ��100%=40%���ʴ�Ϊ��40%��
��100%=40%���ʴ�Ϊ��40%��
(4) �ٳ�����H2SO3��Ka1��1.3��10��2��Ka2��6.6��10��8��һ������Զ���ڶ���������a.������pH=3����������Һ�У�c(H+)��c(HSO3-)��0.001mol/L����c(H2SO3) ��![]() ��10��4 mol/L������Ka2��
��10��4 mol/L������Ka2��![]() ��6.6��10��8����
��6.6��10��8����![]() =
=![]() =
=![]() =6.6��10��5��c(SO32��) ��6.6��10��5��0.001ml/L=6.6��10��8mol/L����a��ȷ��b��H2SO3
=6.6��10��5��c(SO32��) ��6.6��10��5��0.001ml/L=6.6��10��8mol/L����a��ȷ��b��H2SO3![]() H++HSO3-��HSO3-
H++HSO3-��HSO3-![]() H++SO32-��Ka1��
H++SO32-��Ka1��![]() ��1.3��10��2����Ka2��
��1.3��10��2����Ka2��![]() ��6.6��10��8����
��6.6��10��8����![]() =
=![]() =
=![]() =6.6��10��5����b��ȷ��c��δ��֪��Һ���������������Һ����������Ŀ����c����d������Ka1��
=6.6��10��5����b��ȷ��c��δ��֪��Һ���������������Һ����������Ŀ����c����d������Ka1��![]() ��Ka2��
��Ka2��![]() �ı���ʽ��֪����������pH��3��������Һ����Һ�е�c(H+)���䣬c(H2SO3)��c(SO32��)��c(HSO3��)�����ԭ����һ�룬������ʽ��Ȼ������˵��������ĵ���ƽ�ⲻ�ƶ�����d��ȷ����ѡabd��
�ı���ʽ��֪����������pH��3��������Һ����Һ�е�c(H+)���䣬c(H2SO3)��c(SO32��)��c(HSO3��)�����ԭ����һ�룬������ʽ��Ȼ������˵��������ĵ���ƽ�ⲻ�ƶ�����d��ȷ����ѡabd��
��1mol�������Ƶ�ϡ��Һ��������SO2�����������������ƣ��ų�����ΪQkJ����÷�Ӧ���Ȼ�ѧ����ʽΪNaOH(aq)��SO2(g)��NaHSO3(aq) ��H����QkJ/mol���ʴ�Ϊ��NaOH(aq)��SO2(g)��NaHSO3(aq) ��H����QkJ/mol��
��Ka2��![]() ��6.6��10��8��������Һ��c(HSO3��)��c(SO32��)������Һ��c(H+)��6.6��10-8 mol/L���ʴ�Ϊ��6.6��10-8 mol/L��
��6.6��10��8��������Һ��c(HSO3��)��c(SO32��)������Һ��c(H+)��6.6��10-8 mol/L���ʴ�Ϊ��6.6��10-8 mol/L��






