��Ŀ����
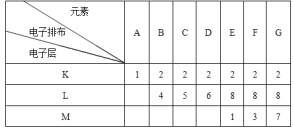
����Ŀ��������A��G����Ԫ�صĵ����Ų�
��������Ԫ�ع��ɵ����ʵĻ�ѧʽ�ش�
(1)д�����ֳ������ɷǼ��Լ��γɵ�˫ԭ�ӷ���_____��
(2)д�������ɼ��Լ��γɵ�˫ԭ�ӷ���___________��
(3)д���ɼ��Լ����ɵ�ֱ���ε���ԭ�ӷ���__��
(4)д������ԭ���Լ��Լ����ɵĿռ乹��ΪV�ε���ԭ�ӷ���__�������ʽΪ___��
(5)д��һ��������ԭ����ɵļȺ����Ӽ��ֺ����ۼ��Ļ�����___�������ʽΪ__________��
(6)д��һ�ּȺ����Ӽ��ֺ��Ǽ��Լ��Ļ�����_____�������ʽΪ______��
���𰸡�H2��N2��O2��Cl2 HCl��CO��NO HCN��CO2��N2O H2O ![]() NaOH
NaOH ![]() Na2O2
Na2O2 ![]()
��������
���ݸ�ԭ�ӵĵ����Ų�����֪��AΪH��BΪC��CΪN��DΪO��EΪNa��FΪAl��GΪCl�������ϼ���Ԫ�أ�д���������������ĸ����ʵĻ�ѧʽ�����ʽ�ȡ�
���ݸ�ԭ�ӵĵ����Ų�����֪��AΪH��BΪC��CΪN��DΪO��EΪNa��FΪAl��GΪCl��
(1) ��ͬ�ַǽ���Ԫ���γɵ�˫ԭ�ӷ����к��зǼ��Լ������ַ��ӷֱ�Ϊ��H2��N2��O2��Cl2������������������ǣ�H2��N2��O2��Cl2��
(2) �����ɼ��Լ��γɵ�˫ԭ�ӷ��ӷֱ�Ϊ��HCl��CO��NO������������������ǣ�HCl��CO��NO��
(3)�ɼ��Լ����ɵ�ֱ���ε���ԭ�ӷ��ӷֱ�ΪHCN��CO2��N2O������������������ǣ�HCN��CO2��N2O��
(4)����ԭ���Լ��Լ����ɵĿռ乹��ΪV�ε���ԭ�ӷ�����H2O�������ʽΪ![]() ���������������������H2O��
���������������������H2O�� ![]() ��
��
(5)������ԭ����ɵļȺ����Ӽ��ֺ����ۼ��Ļ�����NaOH�������ʽΪ![]() ������������������ǣ�NaOH��
������������������ǣ�NaOH��![]() ��
��
(6)�Ⱥ����Ӽ��ֺ��Ǽ��Լ��Ļ�����Na2O2�������ʽΪ![]() ������������������ǣ�Na2O2��
������������������ǣ�Na2O2��![]() ��
��









