ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΘ®1Θ©Α¥“Σ«σ±μ Ψœ¬Ν–”–ΜζΈοΘΚ
ΔΌœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ72«“Ζ–ΒψΉνΒΆΒΡΆιΧΰΒΡΫαΙΙΦρ ΫΘΚ____ΓΘ
ΔΎΥ≥-2-ΕΓœ©ΒΡΫαΙΙΦρ ΫΘΚ____________ΓΘ
ΔέΡ≥»≤ΧΰΨ≠¥ΏΜ·Φ”«βΚσΩ…ΒΟΒΫ2-ΦΉΜυΕΓΆιΘ§‘ρΗΟ»≤ΧΰΒΡΟϊ≥Τ «Θ®Α¥œΒΆ≥ΟϋΟϊΖ®ΟϋΟϊΘ©ΘΚ______ΓΘ
Θ®2Θ©Ρ≥”–ΜζΈοXΖ÷Ή”÷–÷ΜΚ§CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΘ§œύΕ‘Ζ÷Ή”÷ ΝΩ–Γ”Ύ110Θ§Τδ÷–―θ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΈΣ14.8%ΓΘ“―÷ΣΗΟΈο÷ Ω…”κFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§‘ρXΒΡΖ÷Ή” ΫΈΣ_____ΘΜ»τ1molX”κ≈®δεΥ°Ζ¥”Π ±Ω…œϊΚΡ3molBr2Θ§‘ρXΒΡΫαΙΙΦρ ΫΈΣ_____ΓΘ
Θ®3Θ©Α¥“Σ«σ–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ Β―ι “÷Τ±Η““»≤ΒΡΜ·―ßΖΫ≥Χ ΫΘΚ__________ΓΘ
ΔΎ2-δε±ϊΆι”κ«β―θΜ·ΡΤΥ°»ή“ΚΙ≤»»ΒΡΜ·―ßΖΫ≥Χ ΫΘΚ_____ΓΘ
ΓΨ¥πΑΗΓΩC(CH3)4 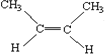 3-ΦΉΜυ-1-ΕΓ»≤ C7H8O
3-ΦΉΜυ-1-ΕΓ»≤ C7H8O  CaC2+2H2OΓζCa(OH)2+C2H2Γϋ CH3CH(Br)CH3+NaOH
CaC2+2H2OΓζCa(OH)2+C2H2Γϋ CH3CH(Br)CH3+NaOH![]() CH3CH(OH)CH3+NaBr
CH3CH(OH)CH3+NaBr
ΓΨΫβΈωΓΩ
(1)ΔΌΆιΧΰ÷ΜΚ§CΓΔHΝΫ÷÷‘ΣΥΊΒΡ±ΞΚΆΧΰΘΜ‘ΎΆιΧΰ÷–CΒΡ ΐΡΩ‘ΫΕύΘ§Ζ–Βψ‘ΫΗΏΘ§÷ßΝ¥‘ΫΕύΘ§Ζ–Βψ‘ΫΒΆΘ§œύΕ‘Ζ÷Ή”÷ ΝΩΈΣ72Θ§ΥΒΟςΚ§”–5ΗωCΓΔ12ΗωHΘ§ΤδΫαΙΙΦρ ΫΘΚC(CH3)4Θ§Ι ¥πΑΗΈΣΘΚC(CH3)4ΘΜ
ΔΎΕΓœ©Κ§”–4ΗωΧΦΘ§«“”–ΧΦΧΦΥΪΦϋΘ§Υ≥-2-ΕΓœ©ΒΡΫαΙΙΦρ ΫΘΚ Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ ΘΜ
ΘΜ
Δέ»≤ΧΰΚ§”–ΧΦΧΦ»ΐΦϋΘ§Ψ≠¥ΏΜ·Φ”«βΚσΩ…ΒΟΒΫ2-ΦΉΜυΕΓΆιΘ§‘ρΗΟ»≤ΧΰΒΡΟϊ≥ΤΈΣΘΚ3-ΦΉΜυ-1-ΕΓ»≤Θ§Ι ¥πΑΗΈΣΘΚ3-ΦΉΜυ-1-ΕΓ»≤ΓΘ
(2) ”–ΜζΈοXΖ÷Ή”÷–÷ΜΚ§CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΘ§œύΕ‘Ζ÷Ή”÷ ΝΩ–Γ”Ύ110Θ§Τδ÷–Κ§―θΒΡ÷ ΝΩΖ÷ ΐΈΣ14.8%Θ§Ζ÷Ή”÷–―θ‘≠Ή”Ήν¥σ ΐΡΩ–Γ”Ύ![]() Θ§Ι X÷–―θ‘≠Ή” ΐΡΩΈΣ1Θ§MΘ®XΘ©=
Θ§Ι X÷–―θ‘≠Ή” ΐΡΩΈΣ1Θ§MΘ®XΘ©=![]() Θ§ΗΟΈο÷ Ω…”κFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§ΥΒΟςΚ§”–Ζ”τ«ΜυΘ§ΦΌ…ηΈΣΕΰ‘Σ»Γ¥ζΘ§Φθ»Ξ1Ηω-C6H4-ΓΔ1Ηω-OHΚσΘ§ Θ”ύΜυΆ≈ΒΡ ΫΝΩΈΣΘΚ108-76-17=15Θ§‘ρ Θ”ύΜυΆ≈ΈΣ-CH3Θ§Ι XΒΡΖ÷Ή” ΫΈΣC7H8OΘ§‘ρXΈΣΦΉΜυ±ΫΖ”ΘΜ»τ1molX”κ≈®δεΥ°Ζ¥”Π ±Ω…œϊΚΡ3molBr2Θ§δεΖΔ…ζΖ”τ«ΜυΒΡΝΎΓΔΕ‘ΈΜ»Γ¥ζΘ§Ι X÷–ΦΉΜυ”κΖ”τ«Μυ¥Π”ΎΦδΈΜΈΜ÷ΟΘ§‘ρXΒΡΫαΙΙΦρ ΫΈΣΘΚ
Θ§ΗΟΈο÷ Ω…”κFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘ§ΥΒΟςΚ§”–Ζ”τ«ΜυΘ§ΦΌ…ηΈΣΕΰ‘Σ»Γ¥ζΘ§Φθ»Ξ1Ηω-C6H4-ΓΔ1Ηω-OHΚσΘ§ Θ”ύΜυΆ≈ΒΡ ΫΝΩΈΣΘΚ108-76-17=15Θ§‘ρ Θ”ύΜυΆ≈ΈΣ-CH3Θ§Ι XΒΡΖ÷Ή” ΫΈΣC7H8OΘ§‘ρXΈΣΦΉΜυ±ΫΖ”ΘΜ»τ1molX”κ≈®δεΥ°Ζ¥”Π ±Ω…œϊΚΡ3molBr2Θ§δεΖΔ…ζΖ”τ«ΜυΒΡΝΎΓΔΕ‘ΈΜ»Γ¥ζΘ§Ι X÷–ΦΉΜυ”κΖ”τ«Μυ¥Π”ΎΦδΈΜΈΜ÷ΟΘ§‘ρXΒΡΫαΙΙΦρ ΫΈΣΘΚ Θ§Ι ¥πΑΗΈΣΘΚC7H8OΘΜ
Θ§Ι ¥πΑΗΈΣΘΚC7H8OΘΜ ΘΜ
ΘΜ
(3) ΔΌ Β―ι “”ΟΒγ ·ΚΆΥ°÷Τ±Η““»≤Θ§ΤδΜ·―ßΖΫ≥Χ ΫΘΚCaC2+2H2OΓζCa(OH)2+C2H2ΓϋΘ§Ι ¥πΑΗΈΣΘΚCaC2+2H2OΓζCa(OH)2+C2H2ΓϋΘΜ
ΔΎ2-δε±ϊΆι”κ«β―θΜ·ΡΤΥ°»ή“ΚΙ≤»»Θ§δε‘≠Ή”±Μτ«Μυ»Γ¥ζΘ§ΤδΜ·―ßΖΫ≥Χ ΫΘΚCH3CH(Br)CH3+NaOH![]() CH3CH(OH)CH3+NaBrΘ§Ι ¥πΑΗΈΣΘΚCH3CH(Br)CH3+NaOH
CH3CH(OH)CH3+NaBrΘ§Ι ¥πΑΗΈΣΘΚCH3CH(Br)CH3+NaOH![]() CH3CH(OH)CH3+NaBrΓΘ
CH3CH(OH)CH3+NaBrΓΘ

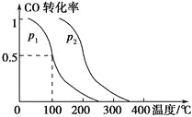
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ1LΚψ»ίΟή±’»ίΤς÷–Φ”»κlmolΒΡN2(g)ΚΆ3molH2(g)ΖΔ…ζΖ¥”ΠΘΚN2(g)+3H2(g) ![]() 2NH3(g) ΓςH<0Θ§ NH3ΒΡΈο÷ ΒΡΝΩ”κ ±ΦδΒΡΙΊœΒ»γœ¬±μΥυ ΨΘ§œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «( )
2NH3(g) ΓςH<0Θ§ NH3ΒΡΈο÷ ΒΡΝΩ”κ ±ΦδΒΡΙΊœΒ»γœ¬±μΥυ ΨΘ§œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «( )
±Φδ(min) | 0 | t1 | t2 | t3 |
NH3Έο÷ ΒΡΝΩ(mol) | 0 | 0.2 | 0.3 | 0.3 |
A. 0t1minΘ§v(NH3)=0.2/t1molΓΛL-1ΓΛmin-1
B. t3 ±‘ΌΦ”»κ1molΒΡN2(g)ΚΆ3molH2(g)Θ§Ζ¥”Π¥ο–¬ΤΫΚβ ±Θ§c(N2)>0.85molΓΛL-1
C. N2(g)+3H2(g) ![]() 2NH3(g)ΒΡΜνΜ·Ρή–Γ”Ύ2NH3(g)
2NH3(g)ΒΡΜνΜ·Ρή–Γ”Ύ2NH3(g) ![]() N2(g)+3H2(g)ΒΡΜνΜ·Ρή
N2(g)+3H2(g)ΒΡΜνΜ·Ρή
D. …ΐΗΏΈ¬Ε»Θ§Ω… Ι’ΐΖ¥”ΠΥΌ¬ Φθ–ΓΘ§ΡφΖ¥”ΠΥΌ¬ ‘ω¥σΘ§Ι ΤΫΚβΡφ“Τ