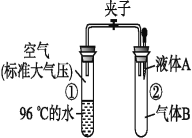
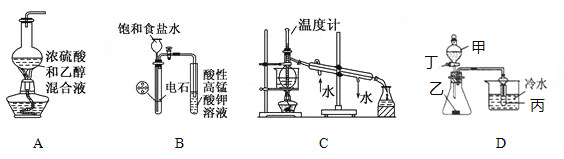
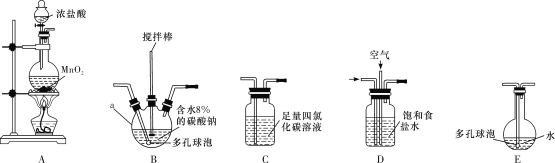
��Ŀ����
����Ŀ��A��B��C��D��E����5��Ԫ�أ�����գ�
��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ�ط���Ϊ______
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ��B��һ�����ӵĽṹʾ��ͼΪ______��C�ĵ����Ų�ʽΪ______
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������D��Ԫ�ط���Ϊ___�����̬ԭ�ӵĵ����Ų�ʽΪ______��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�E��Ԫ�ط���Ϊ____�����̬ԭ�ӵĵ����Ų�ʽΪ______��
���𰸡� N ![]() 1s22s22p63s23p64s1 Fe 1s22s22p63s23p63d64s2��[Ar]3d64s2 Cu 1s22s22p63s23p63d104s1��[Ar]3d104s1
1s22s22p63s23p64s1 Fe 1s22s22p63s23p63d64s2��[Ar]3d64s2 Cu 1s22s22p63s23p63d104s1��[Ar]3d104s1
����������1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ����Ԫ��Ϊ��Ԫ�أ���Ԫ�ط���ΪN��
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ����BΪ��Ԫ�ء�CΪ��Ԫ�ء�B��һ�����ӵĽṹʾ��ͼΪ![]() ��C�ĵ����Ų�ʽΪ1s22s22p63s23p64s1��
��C�ĵ����Ų�ʽΪ1s22s22p63s23p64s1��
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ���������DΪ��Ԫ�أ���Ԫ�ط���ΪFe�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ���EΪͭԪ�أ���Ԫ�ط���ΪCu�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1��