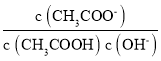ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩν―ΚΆν―ΒΡΚœΫπ“―±ΜΙψΖΚ”Π”Ο”Ύ÷Τ‘λΒγ―ΕΤς≤ΡΓΔ»Υ‘λΙ«ςάΓΔΜ·ΙΛ…η±ΗΓΔΖ…ΜζΒ»ΚΫΧλΚΫΩ’≤ΡΝœΘ§±Μ”ΰΈΣΓΑΈ¥ά¥ άΫγΒΡΫπ τΓ±ΓΘ ‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ν―”–![]() TiΚΆ
TiΚΆ![]() TiΝΫ÷÷‘≠Ή”Θ§ΥϋΟ«ΜΞ≥ΤΈΣ________ΓΘTi‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο «ΒΎ________÷ήΤΎΘ§ΒΎ________ΉεΘΜΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ________Θ§Α¥ΆβΈßΒγΉ”≈≈≤ΦΧΊ’ςTi‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μΖ÷«χ÷– τ”Ύ___________«χ‘ΣΥΊΓΘ
TiΝΫ÷÷‘≠Ή”Θ§ΥϋΟ«ΜΞ≥ΤΈΣ________ΓΘTi‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο «ΒΎ________÷ήΤΎΘ§ΒΎ________ΉεΘΜΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ________Θ§Α¥ΆβΈßΒγΉ”≈≈≤ΦΧΊ’ςTi‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μΖ÷«χ÷– τ”Ύ___________«χ‘ΣΥΊΓΘ
(2)ΤΪν―Υα±Β‘Ύ–Γ–Ά±δ―ΙΤςΓΔΜΑΆ≤ΚΆά©“τΤς÷–ΕΦ”–”Π”ΟΓΘΤΪν―Υα±ΒΨßΧε÷–ΨßΑϊΒΡΫαΙΙ»γΆΦΥυ ΨΘ§ΥϋΒΡΜ·―ß Ϋ «_____________ΓΘ
(3)œ÷”–Κ§Ti3ΘΪΒΡ≈δΚœΈοΘ§Μ·―ß ΫΈΣ[TiCl(H2O)5]Cl2ΓΛH2OΓΘ≈δάκΉ”[TiCl(H2O)5]2ΘΪ÷–Κ§”–ΒΡΜ·―ßΦϋάύ–Ά «________Θ§ΗΟ≈δΚœΈοΒΡ≈δΈΜΧε «________ΓΘ
ΓΨ¥πΑΗΓΩ(1)Ά§ΈΜΥΊ ΥΡ Δτ B 1s22s22p63s23p63d24s2(Μρ[Ar]3d24s2) d BaTiO3 ΦΪ–‘Ι≤ΦέΦϋ(ΜρΙ≤ΦέΦϋ)ΓΔ≈δΈΜΦϋ H2OΓΔClΘ≠
ΓΨΫβΈωΓΩ
Θ®1Θ©![]() TiΚΆ
TiΚΆ![]() TiΒΡ÷ Ή” ΐœύΆ§Θ§÷ ΝΩ ΐΚΆ÷–Ή” ΐ≤ΜΆ§ΘΜTi‘ΣΥΊΒΡΚΥΒγΚ… ΐΈΣ22ΘΜ
TiΒΡ÷ Ή” ΐœύΆ§Θ§÷ ΝΩ ΐΚΆ÷–Ή” ΐ≤ΜΆ§ΘΜTi‘ΣΥΊΒΡΚΥΒγΚ… ΐΈΣ22ΘΜ
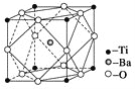
Θ®2Θ©”…ΨßΑϊΫαΙΙΩ…÷ΣΘ§8Ηων―‘≠Ή”ΈΜ”ΎΕΞΒψΘ§ 1Ηω±Β‘≠Ή”ΈΜ”ΎΧε–ΡΘ§ΨßΑϊ÷–‘≠Ή”Ηω ΐΈΣ1Θ§12Ηω―θ‘≠Ή”ΈΣ”Ύάβ…œΘ§”…Ζ÷Χ·Ζ®ΦΤΥψΩ…ΒΟΜ·―ß ΫΘΜ
Θ®3Θ©≈δάκΉ”[TiCl(H2O)5]2+÷–Ti3+ΈΣ÷––ΡάκΉ”Θ§5ΗωH2OΖ÷Ή”ΚΆ1ΗωCl-ΈΣ≈δΈΜΧεΘ§≈δΈΜ ΐΈΣ6ΓΘ
Θ®1Θ©![]() TiΚΆ
TiΚΆ![]() TiΒΡ÷ Ή” ΐœύΆ§Θ§÷ ΝΩ ΐ≤ΜΆ§Θ§÷–Ή” ΐ≤ΜΆ§Θ§ΜΞΈΣΆ§ΈΜΥΊΘΜTi‘ΣΥΊΒΡΚΥΒγΚ… ΐΈΣ22Θ§ΈΜ”Ύ‘ΣΥΊ÷ήΤΎ±μΒΎΥΡ÷ήΤΎΔτ BΉεΘ§ΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p63s23p63d24s2(Μρ[Ar]3d24s2)Θ§Α¥ΆβΈßΒγΉ”≈≈≤ΦΧΊ’ςTi‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μΖ÷«χ÷– τ”Ύd«χ‘ΣΥΊΘΜ
TiΒΡ÷ Ή” ΐœύΆ§Θ§÷ ΝΩ ΐ≤ΜΆ§Θ§÷–Ή” ΐ≤ΜΆ§Θ§ΜΞΈΣΆ§ΈΜΥΊΘΜTi‘ΣΥΊΒΡΚΥΒγΚ… ΐΈΣ22Θ§ΈΜ”Ύ‘ΣΥΊ÷ήΤΎ±μΒΎΥΡ÷ήΤΎΔτ BΉεΘ§ΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p63s23p63d24s2(Μρ[Ar]3d24s2)Θ§Α¥ΆβΈßΒγΉ”≈≈≤ΦΧΊ’ςTi‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μΖ÷«χ÷– τ”Ύd«χ‘ΣΥΊΘΜ
Θ®2Θ©”…ΨßΑϊΫαΙΙΩ…÷ΣΘ§8Ηων―‘≠Ή”ΈΜ”ΎΕΞΒψΘ§ΨßΑϊ÷–‘≠Ή”Ηω ΐΈΣ8ΓΝ![]() =1Θ§1Ηω±Β‘≠Ή”ΈΜ”ΎΧε–ΡΘ§ΨßΑϊ÷–‘≠Ή”Ηω ΐΈΣ1Θ§12Ηω―θ‘≠Ή”ΈΣ”Ύάβ…œΘ§ΨßΑϊ÷–‘≠Ή”Ηω ΐΈΣ12ΓΝ
=1Θ§1Ηω±Β‘≠Ή”ΈΜ”ΎΧε–ΡΘ§ΨßΑϊ÷–‘≠Ή”Ηω ΐΈΣ1Θ§12Ηω―θ‘≠Ή”ΈΣ”Ύάβ…œΘ§ΨßΑϊ÷–‘≠Ή”Ηω ΐΈΣ12ΓΝ![]() =3Θ§ν―ΓΔ±ΒΓΔ―θΒΡ‘≠Ή”Ηω ΐ±»ΈΣ1:1:3Θ§‘ρΜ·―ß ΫΈΣBaTiO3ΘΜ
=3Θ§ν―ΓΔ±ΒΓΔ―θΒΡ‘≠Ή”Ηω ΐ±»ΈΣ1:1:3Θ§‘ρΜ·―ß ΫΈΣBaTiO3ΘΜ
Θ®3Θ©≈δάκΉ”[TiCl(H2O)5]2+÷–Ti3ΘΪΈΣ÷––ΡάκΉ”Θ§5ΗωH2OΖ÷Ή”ΚΆ1ΗωCl-ΈΣ≈δΈΜΧεΘ§≈δΈΜ ΐΈΣ6Θ§÷––ΡάκΉ”ΚΆ≈δΈΜΧε÷°Φδ“‘≈δΈΜΦϋΫαΚœΘ§Υ°Ζ÷Ή”÷–―θ‘≠Ή”ΚΆ«β‘≠Ή”–Έ≥…ΦΪ–‘Ι≤ΦέΦϋΓΘ

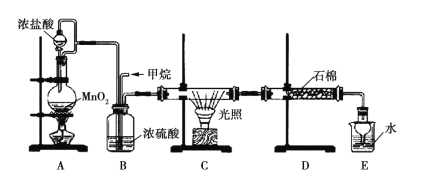
ΓΨΧβΡΩΓΩΡ≥Έ¬Ε»œ¬Θ§H2(g)ΘΪCO2(g)![]() H2O(g)ΘΪCO2(g)ΒΡΤΫΚβ≥Θ ΐ
H2O(g)ΘΪCO2(g)ΒΡΤΫΚβ≥Θ ΐ![]() ΓΘΗΟΈ¬Ε»œ¬‘ΎΦΉΓΔ““ΓΔ±ϊ»ΐΗωΚψ»ίΟή±’»ίΤς÷–Θ§
ΓΘΗΟΈ¬Ε»œ¬‘ΎΦΉΓΔ““ΓΔ±ϊ»ΐΗωΚψ»ίΟή±’»ίΤς÷–Θ§
Τπ Φ≈®Ε» | ΦΉ | ““ | ±ϊ |
c(H2)/mol | 0.010 | 0.020 | 0.020 |
c(CO2)/mol | 0.010 | 0.010 | 0.020 |
ΆΕ»κH2(g)ΚΆCO2(g)Θ§ΤδΤπ Φ≈®Ε»»γ±μΥυ ΨΓΘœ¬Ν–≈–Εœ≤Μ’ΐ»ΖΒΡ «Θ® Θ©
A.ΤΫΚβ ±Θ§““÷–CO2ΒΡΉΣΜ·¬ ¥σ”Ύ60%
B.ΤΫΚβ ±Θ§ΦΉ÷–ΚΆ±ϊ÷–H2ΒΡΉΣΜ·¬ Ψυ «60ΘΞ
C.ΤΫΚβ ±Θ§±ϊ÷–c(CO2) «ΦΉ÷–ΒΡ2±ΕΘ§ «0.012mol/L
D.Ζ¥”ΠΩΣ Φ ±Θ§±ϊ÷–ΒΡΖ¥”ΠΥΌ¬ ΉνΩλΘ§ΦΉ÷–ΒΡΖ¥”ΠΥΌ¬ Ήν¬ΐ
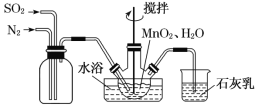
ΓΨΧβΡΩΓΩCl2ΓΔΤ·ΑΉ“Κ(”––ß≥…Ζ÷ΈΣ NaClO)‘Ύ…ζ≤ζΓΔ…ζΜν÷–ΙψΖΚ”Ο”Ύ…±ΨζΓΔœϊΕΨΓΘ
(1)ΒγΫβ NaCl »ή“Κ…ζ≥…Cl2ΒΡΜ·―ßΖΫ≥Χ Ϋ «________________ΓΘ
(2)Cl2»ή”ΎH2OΓΔNaOH »ή“ΚΦ¥ΜώΒΟ¬»Υ°ΓΔΤ·ΑΉ“ΚΓΘ
ΔΌΗ…‘οΒΡ¬»Τχ≤ΜΡήΤ·ΑΉΈο÷ Θ§ΒΪ¬»Υ°»¥”–Τ·ΑΉΉς”ΟΘ§ΥΒΟςΤπΤ·ΑΉΉς”ΟΒΡΈο÷ «_____ΓΘ
ΔΎ25ΓφΘ§Cl2”κH2OΓΔNaOH ΒΡΖ¥”Π»γœ¬ΘΚ
Ζ¥”ΠΔώ | Cl2+H2O |
Ζ¥”ΠΔρ | Cl2+2OH- |
Ϋβ Ά≤Μ÷±Ϋ” Ι”Ο¬»Υ°Εχ Ι”ΟΤ·ΑΉ“ΚΉωœϊΕΨΦΝΒΡ‘≠“ρ_____ΓΘ
(3)Φ“ΆΞ Ι”ΟΤ·ΑΉ“Κ ±Θ§≤Μ“Υ÷±Ϋ”Ϋ”¥ΞΧζ÷ΤΤΖΘ§Τ·ΑΉ“ΚΗ· ¥ΧζΒΡΒγΦΪΖ¥”ΠΈΣΘΚFe-2e-=Fe2+ΘΜClO-ΖΔ…ζΒΡΒγΦΪΖ¥”Π ΫΈΣ____________ΓΘ
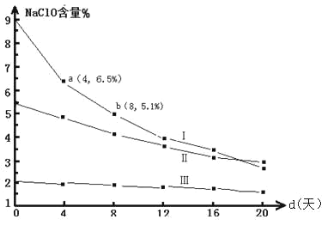
(4)―–ΨΩΤ·ΑΉ“ΚΒΡΈ»Ε®–‘Ε‘Τδ…ζ≤ζΚΆ±Θ¥φ”– ΒΦ “β“εΓΘ30Γφ ±Θ§pH=11 ΒΡΤ·ΑΉ“Κ÷–NaClO ΒΡ÷ ΝΩΑΌΖ÷Κ§ΝΩΥφ ±Φδ±δΜ·»γœ¬ΘΚ
±»ΫœΖ÷ΫβΥΌ¬ v(I)ΓΔ v(II)ΒΡ¥σ–ΓΙΊœΒ_____Θ§‘≠“ρ «_____ΓΘ