��Ŀ����
����Ŀ����.�ҹ����ຣʡ�������κ�ʢ��ʳ�Σ�������ʳ�ι�ϵ���У�ʳ�����ϰ���������ִ����Ĺ�ũҵ�����о�����Ҫ���á������к�Ca2����Mg2����SO42���Լ���ɳ�����ʣ�Ϊ�˳�ȥ���������ʣ�������ʵ�鲽������ᴿ��
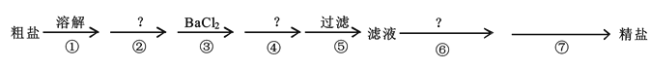
��1���ܲ������Լ���____��
��2���ڢ�������Ӧ�����ӷ���ʽΪ____��____��
��3���ڢ߲��IJ�������____����Ҫ�IJ���������____��____��
��4��ͨ��������й��˺����Һ������SO42���ѳ����IJ���������____��
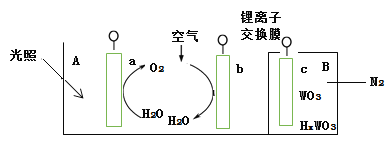
��.��1���谢���ӵ�����ΪNA����״���£�ijO2��N2�Ļ������m g����b�����ӣ���n g�û����������ͬ״������ռ�������____L��
��2����xR2��+yH��+O2�TmR3��+nH2O�����ӷ���ʽ�У��Ի�ѧ������m��R2����R3���ж���ȷ����____��
A��m=y��R3������������ B��m=2y��R2��������
C��m=2��R3���������� D��m=4��R2���ǻ�ԭ��
��3����˫���ŷ�������з�Ӧ�ĵ���ת�Ʒ������Ŀ____��2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O
���𰸡�Na2CO3��Һ 2H��+ CO32��= CO2����H2O H�� +OH��= H2O �����ᾧ �ƾ��� ������ ȡ��������Һ���Թ��У������μ�BaCl2��Һ����û������˵��SO42���ѳ��� ![]() AD
AD 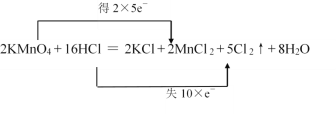
��������
��.�ڴ����ᴿʱ��̼���Ƽ����Ȼ����ĺ��棬��������ֻҪ�ڹ���֮ǰ���ɣ�Ҫ�����һ���������ᣬ���ݳ���ԭ���������
��.��1���ȸ���n��![]() �����mg�����������ʵ�����Ȼ�����M��
�����mg�����������ʵ�����Ȼ�����M��![]() �������������ƽ��Ħ��������������V��n
�������������ƽ��Ħ��������������V��n![]() Vm��
Vm��![]() ��22.4L/mol��������bg�������������
��22.4L/mol��������bg�������������
��2����xR2��+yH��+O2�TmR3��+nH2O�����ӷ���ʽ�У�����Oԭ���غ��֪n��2��Ȼ�����Hԭ���غ��֪y��4���ٽ�ϵ���غ㡢ԭ���غ��֪x��m��4������ϻ��ϼ۱仯�ж�����������ԭ�����ɣ�
��3��ʹ��˫���ŷ�ʱһ��Ҫע���ͷ���˶�Ӧ��Ӧ��Ԫ�أ����ҵ�ʧ�����غ㡣
��.��1���ڴ����ᴿʱ��Ҫ�����һ���������ᣬ��ȥ���������������Ӻ�̼������ӣ�̼���Ƽ����Ȼ����ĺ��棬��ȥ�����Ӻͼ���Ĺ����ı����ӣ�NaOH��Һ��þ���ӣ�˳���ڹ���ǰ���ɣ����Ԣڲ�����NaOH��Һ���ܲ������Լ���Na2CO3��Һ���ʴ�Ϊ��Na2CO3��Һ��
��2���ڢ��������ϡ���ᣬ��ȥ���������������Ӻ�̼������ӣ���ص����ӷ���ʽΪ��2H��+ CO32��= CO2����H2O��H��+OH��= H2O���ʴ�Ϊ��2H��+ CO32��= CO2����H2O��H��+OH��= H2O��
��3���Ȼ�����Һ���������ᾧ���Եõ����Σ���Ҫ�IJ��������оƾ��ơ��������ȣ��ʴ�Ϊ�������ᾧ���ƾ��ƣ���������
��4����Һ������������Ƿ��������ͨ���μӹ����Ȼ��������жϣ�������������Ϊ��ȡ��������Һ���Թ��У������μ�BaCl2��Һ����û������˵��SO42���ѳ��������Թ��е���Һ���ֻ�����֤��SO42δ�������ʴ�Ϊ��ȡ��������Һ���Թ��У������μ�BaCl2��Һ����û������˵��SO42���ѳ�����
��. (1)��״����O2��N2�Ļ������mg����b�����ӣ�mg�û����������ʵ���Ϊ��![]() ��
��![]() mol���û�������ƽ��Ħ������Ϊ��
mol���û�������ƽ��Ħ������Ϊ��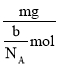 ��
��![]() g/mol����ng�û�������ڱ�״������ռ�����Ϊ��V��n
g/mol����ng�û�������ڱ�״������ռ�����Ϊ��V��n![]() Vm��
Vm��![]() ��22.4L/mol=
��22.4L/mol= ��22.4L/mol��
��22.4L/mol��![]() L���ʴ�Ϊ��
L���ʴ�Ϊ��![]() ��
��
(2)��ӦxR2��+yH��+O2�TmR3��+nH2O�У�����Oԭ���غ��֪n��2��Ȼ�����Hԭ���غ��֪y��4���ٽ�ϵ���غ㡢ԭ���غ��֪��x��m��2x��4��3m�����x��m�T4����÷�ӦΪ��4R2����4H����O2��4R3����2H2O��
A.���ݷ�����֪��m��y��4��R3��Ϊ���������A��ȷ��
B.�÷�Ӧ��R2�����ϼ����߱���������m��y��4����B����
C.�÷�Ӧ��R2�����ϼ����߱�O2������R3����R3��Ϊ���������C����
D.���ݷ�����֪��m��4��R2�����ϼ����߱�������Ϊ��ԭ������D��ȷ��
�ʴ�ѡAD��
(3)��˫���ŷ���ʾ����ת��Ҫע�������ϼ۱仯Ԫ�غ͵�ʧ�����غ㣬2KMnO4+16HCl��Ũ���T2KCl+2MnCl2+5Cl2��+8H2O��˫���ŷ��ɱ�ʾΪ��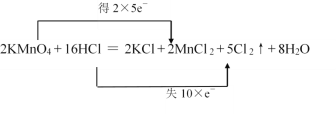 ���ʴ�Ϊ��
���ʴ�Ϊ��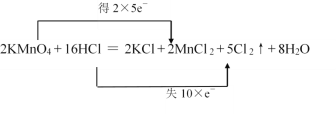 ��
��