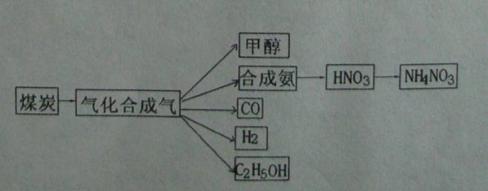
��Ŀ����
��7�֣�830Kʱ�����ܱ������з������п��淴Ӧ��CO��g��+H2O��g��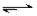 CO2��g��+H2��g�� ��H��0�Իش��������⣺
CO2��g��+H2��g�� ��H��0�Իش��������⣺
��1������ʼʱc��CO��=2mol��L-1��c��H2O��=3mol��L-1���ﵽƽ��ʱCO��ת����Ϊ60%�����ڸ��¶��£��÷�Ӧ��ƽ�ⳣ��K= ��
��2������ͬ�¶��£�����ʼʱc��CO��=1mol��L-1��c��H2O��=2mol��L-1����Ӧ����һ��ʱ����H2��Ũ��Ϊ0.5 mol��L-1�����ʱ�÷�Ӧ�Ƿ�ﵽƽ��״̬ ����ǡ��롰������ʱv������ v���棩������ڡ���С�ڡ����ڡ��������жϵ������� ��
��3���������¶ȣ��÷�Ӧ��Kֵ�� ���÷�Ӧ�Ļ�ѧ��Ӧ���ʽ� �����������С�����䡱����
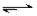 CO2��g��+H2��g�� ��H��0�Իش��������⣺
CO2��g��+H2��g�� ��H��0�Իش��������⣺��1������ʼʱc��CO��=2mol��L-1��c��H2O��=3mol��L-1���ﵽƽ��ʱCO��ת����Ϊ60%�����ڸ��¶��£��÷�Ӧ��ƽ�ⳣ��K= ��
��2������ͬ�¶��£�����ʼʱc��CO��=1mol��L-1��c��H2O��=2mol��L-1����Ӧ����һ��ʱ����H2��Ũ��Ϊ0.5 mol��L-1�����ʱ�÷�Ӧ�Ƿ�ﵽƽ��״̬ ����ǡ��롰������ʱv������ v���棩������ڡ���С�ڡ����ڡ��������жϵ������� ��
��3���������¶ȣ��÷�Ӧ��Kֵ�� ���÷�Ӧ�Ļ�ѧ��Ӧ���ʽ� �����������С�����䡱����
��7�֣���1��1 ��2������ ��Ϊ
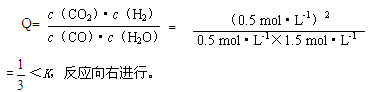
��3������ ��С
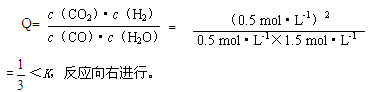
��3������ ��С
(1)����ƽ������������������֪��ƽ��ʱCO��Ũ��Ϊ0.8mol/L,H2OŨ��Ϊ1.8mol/L,CO2Ũ��Ϊ1.2mol/L,H2��Ũ��Ϊ1.2mol/L.�ٸ���Kֵ���㹫ʽ�õ�:K=1.
(2)��0.5mol/LH2������,Kֵ��С��1��,����û�дﵽƽ��״̬,����KֵС��1,��˵�������Ũ�Ȼ���Ҫ���,��Ӧ��Ӧ�����������,V����V��.
(3)�÷�Ӧ��һ�����ȷ�Ӧ,�����¶�,ƽ�������ƶ�,������Ũ������,��Ӧ��Ũ�Ƚ���,Kֵ���.���ǽ����¶Ȼ�ʹ�����˶����ʼ���,��ѧ��Ӧ����Ҳ���С.

��ϰ��ϵ�д�
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
�����Ŀ
 2Z��g�� ��H����a kJ��mol��1��a��0������һ���ݻ��̶��������м���2molX2��1mo1Y2����500��Գ�ַ�Ӧ��ƽ���Z��Ũ��ΪW mol��L��1���ų�����b kJ��
2Z��g�� ��H����a kJ��mol��1��a��0������һ���ݻ��̶��������м���2molX2��1mo1Y2����500��Գ�ַ�Ӧ��ƽ���Z��Ũ��ΪW mol��L��1���ų�����b kJ�� ��X2����2
��X2����2 ��Y2�� d�������ڵ��ܶȱ��ֲ���
��Y2�� d�������ڵ��ܶȱ��ֲ��� 2Z(g)������ӦΪ���ȷ�Ӧ����Ϊ��ʹƽ��������Z�ķ����ƶ���Ӧѡ�����е����������ǣ� ��
2Z(g)������ӦΪ���ȷ�Ӧ����Ϊ��ʹƽ��������Z�ķ����ƶ���Ӧѡ�����е����������ǣ� ��
 4C��g��+D��g����Ӧ10min���ƽ�⣬��ʱD��Ũ��Ϊ0.5mol/L������˵����ȷ����
4C��g��+D��g����Ӧ10min���ƽ�⣬��ʱD��Ũ��Ϊ0.5mol/L������˵����ȷ����  A��ת����һ������50%
A��ת����һ������50%
 2C0(g) ��H>O��K1 �� ��
2C0(g) ��H>O��K1 �� ��