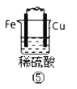
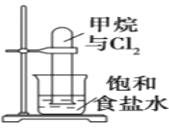
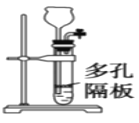
��Ŀ����
����Ŀ����ͼ��Ԫ�����ڱ���һ���֣����ݢ١��������ڱ��е�λ�ð���ĿҪ��ش�
�� ���� | ��A | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� |
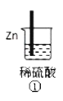
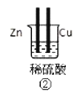
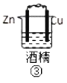
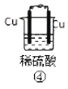
(1)д������⻯��͢ڵ��⻯�ﷴӦ�Ļ�ѧ����ʽ________��
(2)��Ԫ�آ١����У��ǽ�������ǿ��Ԫ����________����Ԫ�����ƣ���
(3)д��Ԫ�آڵ�����������Ӧˮ����ĵ��뷽��ʽ________��
(4)д���ݢ�����������ˮ����֮������ӷ�Ӧ________________��
(5)�ɢں͢���ɵĻ������и�ԭ�Ӿ������ȶ��ṹ��д���û�����Ľṹʽ________________��
���𰸡�![]() ��
�� ![]()
![]()
��������
����Ԫ�������ڱ��е�λ�ã�����֪����ΪH����ΪN����ΪO����ΪF����ΪNa����ΪMg����ΪAl����ΪCl���Դ˽��
��1��HClΪ���Ի����NH3Ϊ�������壬���߷�Ӧ�ķ���ʽΪ��![]() ��
��
��2��Ԫ�����ڱ��У���������Ԫ�طǽ����������ӣ���������Ԫ�طǽ�������С����˷ǽ�����ǿ��Ϊ����
��3��NԪ�ص�����������Ӧˮ����ΪHNO3������ǿ����ʣ�����뷽��ʽΪ��![]() ��
��
��4��NaԪ������������ˮ����ΪNaOH������ǿ�ClԪ������������ˮ����ΪHClO4������ǿ�ᣬ���߷�Ӧ�����ӷ���ʽΪ��![]() ��
��
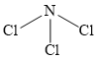
��5��Nԭ���������5�����ӣ�Clԭ���������7�����ӣ�����ɵĻ������и�ԭ�Ӿ������ȶ��ṹ���������ΪNCl3�����ڹ��ۻ����Nԭ���ϴ���1���¶Ե��Ӷԣ����NCl3�Ŀռ�ṹΪ�����Σ���ṹʽΪ�� ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�