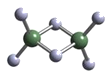
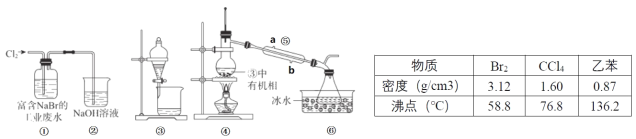
��Ŀ����
����Ŀ�����мס��ҡ����dz������л��������Ҫ��ش��������⣺
�ף�CH3CH=CH2 �ң�![]() ����HOCH2CH2CH2COOH
����HOCH2CH2CH2COOH
(1)��������_______��
(2)������������Ȼ�̼��Һ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ______��
(3)��Ҳ�ܷ����ۺϷ�Ӧ������Ľṹ��ʽΪ_____________��
(4)����Ũ�����ϼ��ȣ�������Ԫ�����ʵĻ�ѧ��Ӧ����ʽ��____________��
(5)д���ҵ�ͬ���칹���У����������Һ˴Ź���������2��壬�������Ϊ3��2�Ľṹ��ʽΪ__________��
���𰸡���ϩ CH3CH= CH2 +Br2![]() CH3CHBrCH2Br
CH3CHBrCH2Br 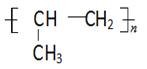 HOCH2CH2CH2COOH
HOCH2CH2CH2COOH![]()
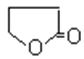 +H2O
+H2O ![]()
��������
(1)�ɼĽṹ��ʽ����ϳ������ʵ�������ʽ������
(2)����̼̼˫���������巢���ӳɷ�Ӧ��
(3)����̼̼˫�����ɷ����Ӿ۷�Ӧ���ɸ߾��
(4)�������ǻ����Ȼ����ɷ���������������Ӧ���ɻ�״����
(5)��Ϊ�ұ�(![]() )���ҵ�ͬ���칹���У����б�����Ϊ���ױ�����Ϻ˴Ź����������жϡ�
)���ҵ�ͬ���칹���У����б�����Ϊ���ױ�����Ϻ˴Ź����������жϡ�
(1)�ɽṹ��ʽ��֪��Ϊ��ϩ���ʴ�Ϊ����ϩ��
(2)��(CH3CH=CH2)�к���̼̼˫���������巢���ӳɷ�Ӧ����Ӧ�ķ���ʽΪCH3CH=CH2+Br2��CH3CHBrCH2Br���ʴ�Ϊ��CH3CH=CH2+Br2��CH3CHBrCH2Br��
(3)��(CH3CH=CH2)�к���̼̼˫�����ɷ����Ӿ۷�Ӧ���ɸ߾������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(4)��(HOCH2CH2CH2COOH)�к����ǻ����Ȼ����ɷ���������������Ӧ���ɻ�״����������Ԫ���ķ�ӦΪHOCH2CH2CH2COOH![]()
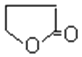 +H2O���ʴ�Ϊ��HOCH2CH2CH2COOH
+H2O���ʴ�Ϊ��HOCH2CH2CH2COOH![]()
 +H2O��
+H2O��
(5)��Ϊ�ұ�(![]() )���ҵ�ͬ���칹���У����б�������ͬ���칹��Ӧ��Ϊ���ױ������ڡ��䡢��3�֣����к˴Ź���������2��壬�������Ϊ3��2�Ľṹ��ʽΪ
)���ҵ�ͬ���칹���У����б�������ͬ���칹��Ӧ��Ϊ���ױ������ڡ��䡢��3�֣����к˴Ź���������2��壬�������Ϊ3��2�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ![]() ��
��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�