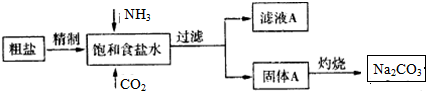
��Ŀ����
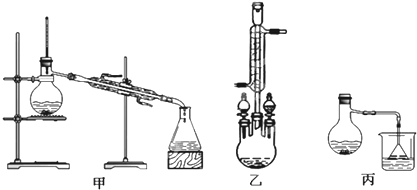
9��ij��ѧС�������ͼװ�ã��Ի������Ʊ�����ϩ����֪

| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
����ͼ1����B���˵�������е�������������
�ڼ����Թ�A������ˮԡ������ֱ�Ӽ��ȣ�Ŀ���ǿ����¶�85�����ң���ֹ�������ӷ���ʹ���Ⱦ��ȣ��Թ�C���ڱ�ˮԡ�е�Ŀ���Ƿ�ֹ����ϩ�ӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ�
���뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ� �㣨��ϡ����¡�������Һ����c�������ţ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��ͼ2װ��������ȴˮ��g�ڽ��루����ĸ����
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����c��
A������ʱ��70�濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���ƻ���ϩʱ������Ũ�����ǿ�����ԣ�����������SO2��CO2��ˮ��������С���������Լ�����
 ��SO2��CO2��ˮ�������������ͨ���Լ���˳����
��SO2��CO2��ˮ�������������ͨ���Լ���˳�����ܢݢ٢ݢڢۣ���ܢݢ٢ݢۢڣ�������ţ���%2��Na2SO3��Һ ������KMnO4��Һ ��ʯ��ˮ����ˮCuSO4 ��Ʒ����Һ��
���� ������ݴ�����ȥ��Ӧԭ�������û������Ʊ�����ϩ��ʵ��������̣��漰�Ʊ��������ᴿ��ʵ����ۺ����ۣ��Ʊ������з�Ӧ���Һ����ʱΪ�˷�����Ҫ�������Ƭ�������ռ���Ҫ����ʹ֮Һ�������ӷ����ֲ�Ʒ����ʱ�漰����Һ��������ַ�����������̽���˲�Ʒ���ȷ�����
��1�����������ɵĻ���ϩ�ķе�Ϊ83�棬�ӷ���Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ڲ�����ˮԡ��Ŀ����ʹ�Թ����Ⱦ��ȣ����ڿ����¶ȣ���ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
������ʱΪ����������Ч������ȴˮ���¿ڣ�g�����룻
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
a������ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�����
b����ȡ�Ļ���ϩ���ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����
c���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����
��3������ ����������KMnO4��Һ������ˮ����ͭ����ˮ����Ʒ�����������������Ը�����س�ȥ�������������ʯ��ˮ���������̼��
����������KMnO4��Һ������ˮ����ͭ����ˮ����Ʒ�����������������Ը�����س�ȥ�������������ʯ��ˮ���������̼��
��� �⣺��1�����������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ʴ�Ϊ��������
�ڼ����Թ�A������ˮԡ��Ŀ���ǿ����¶�85�����ң���ֹ�������ӷ���ʹ���Ⱦ��ȣ���ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ������ֹ����ϩ�ӷ���
�ʴ�Ϊ�������¶�85�����ң���ֹ�������ӷ���ʹ���Ⱦ��ȣ���ֹ����ϩ�ӷ���
��2���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ����������룺�Ʊ����������ᴿ����ʱ��c������Na2CO3��Һ��ϴ�ӿɳ�ȥ�ᣬ
�ʴ�Ϊ���ϲ㣻c��
������װ��Ҫ�������ܣ�Ϊ����������Ч������ȴˮ�ķ���Ӧ�ú������������෴����ȴˮ���¿ڣ�g�����룬
�ʴ�Ϊ��g��
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棬���ռ���ƷӦ�����¶���83�����ң�
a������ʱ��70�濪ʼ�ռ���Ʒ����ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�������a����
b��������ʵ���������ˣ���ȡ�Ļ���ϩ�����ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�������b����
c�����ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�������c��ȷ����ѡc��
�ʴ�Ϊ��83�棻c��
��3������ ����������KMnO4��Һ������SO2����������KMnO4��Һ��ɫ��Ʒ����Һ��ʯ��ˮ������CO2����ʯ��ˮ������ˮ����������ˮCuSO4�������ڼ���������������迼���Լ���ѡ���˳��ֻҪͨ����Һ���ͻ����ˮ����������ȼ���ˮ������Ȼ�����SO2���ڼ���֮���ȥSO2����SO2�����ñ���Na2SO3��Һ��������CO2�ͱ�ϩ�����˳��Ϊ�ܢݢ٢ݢڢۣ���ܢݢ٢ݢۢڣ���
����������KMnO4��Һ������SO2����������KMnO4��Һ��ɫ��Ʒ����Һ��ʯ��ˮ������CO2����ʯ��ˮ������ˮ����������ˮCuSO4�������ڼ���������������迼���Լ���ѡ���˳��ֻҪͨ����Һ���ͻ����ˮ����������ȼ���ˮ������Ȼ�����SO2���ڼ���֮���ȥSO2����SO2�����ñ���Na2SO3��Һ��������CO2�ͱ�ϩ�����˳��Ϊ�ܢݢ٢ݢڢۣ���ܢݢ٢ݢۢڣ���
�ʴ�Ϊ���ܢݢ٢ݢڢۣ���ܢݢ٢ݢۢڣ���
���� ���⿼���л���ϳɷ�������ƣ���Ŀ�ѶȽϴ��ۺ��Խ�ǿ������ʱע��������ʵķ��롢�ᴿ�������������ʵ����ʵ���ͬ�ǽ�����Ĺؼ���

| A�� | PH=7����Һһ�������� | |
| B�� | ����Һc��H+��=c��OH-��ʱ����Һ������ | |
| C�� | ������Һһ�������ԣ���������Һ���������Ի���� | |
| D�� | ����Һ������ʱ��ˮ�����c��H+����c��OH-�� |
| A�� | 4m mol | B�� | 10m mol | C�� | $\frac{10m}{3}$mol | D�� | $\frac{2m}{3}$mol |
��һ��ԭ�ϡ���Ӧ������ƽ��ת���ʡ��ղ���
| ��Ȳˮ���� | ��ϩ������ | |
| ԭ�� | ��Ȳ��ˮ | ��ϩ������ |
| ��Ӧ���� | HgSO4��100��125�� | PdCl2-CuCl2��100��125�� |
| ƽ��ת���� | ��Ȳƽ��ת����90%���� | ��ϩƽ��ת����80%���� |
| �ղ��� | 2.5�֣�ij�豸�����£� | 3.6�֣���ͬ�豸�����£� |
| ԭ���������չ��� | |
| ��Ȳ | CaCO3$\stackrel{��850-1100��}{��}$CaO$��_{1100��}^{��+C����¯}$CaC2$\stackrel{�۱���ʳ��ˮ}{��}$C2H2 |
| ��ϩ | ��Դ��ʯ���ѽ��� |
��1��д�����л�ѧ����ʽ��
a����Ȳˮ��������ȩCH��CH+H2O$��_{��}^{����}$CH3CHO��
b����ϩ����������ȩ2CH2=CH2+O2$��_{��}^{����}$2CH3CHO��
��2���������з������ִ���ҵ����ϩ��������ȡ����Ȳˮ�������������ܵ�ԭ�ӻ�����ԭ����Դ���ܺĵȽǶȷ�������дһ�㣩�����߷�Ӧ�����¶��൱������Ȳˮ��������ȩʹ�õ��ǹ��δ��������εĶ��Դ���Ȼ��ϩ��������ת������С����Ȳˮ����������Ӧ�졢�ղ�������ߵö࣬��Ȳ����ȡҪ�����ಽ��Ӧ�Ƶã������Ĵ��������ܡ����ܣ�������ϩ��Դ��ʯ���ѽ��������ĵ�����������Ȳ�٣��ҽ�����õȣ�
��3���ӻ�ѧ��Ӧ���ʽǶȷ���������ͬ�����£�������ȡ��ȩ�ķ������ֿ죿��ϩ�������죮
��4�������������ַ����ķ�Ӧ�����������ӡ�100��������ѹ����ԭ��ת���ʻ�������һЩ������ʵ��������ȴ�����������ķ�������������Ϊ���ַ�������ͬ��Ӧ�����£�ת�����Ѿ��ܸߣ�����ѹǿ�����������������豸Ԥ�㣬�����ã�
��5�����ij��������ϩΪԭ�ϣ�ͨ��3�������Ƶþ�����ϩ����д�������ķ�Ӧ����ʽ�����Լ����ܼ�����ѡ���Ĺ����в�����ȡ����Ӧ��
 ��
�� 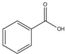 ��Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���
��Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |
��Բ����ƿ�м���12.2g�����ᣨM=122g/mol����20ml�״����ܶ�Լ0.79g•mL-1 ������С�ļ���3mLŨ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
��1������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽC6H5CO18OH+CH3OH$?_{��}^{ŨH_{2}SO_{4}}$C6H5COOCH3+H218O��Ũ����������ǣ���������ˮ����
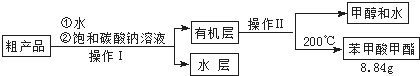
��2���ס��ҡ�����λͬѧ�ֱ����������ͼ����ʵ���Һϳɱ����������װ�ã��г������ͼ�������������ȥ���������л���ķе㣬��ò�����װ�ã���ס����ҡ���������
�ֲ�Ʒ�ľ���
��3������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ��������������̽��о��ƣ����������ͼд���������������ƣ��������Һ ����������
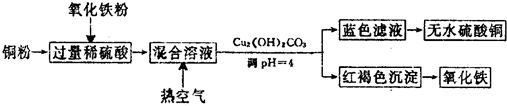
��4���ܷ���NaOH��Һ���汥��̼������Һ������ܡ�����������ԭ������������ǿ��ٽ������������ˮ�⣬���²�Ʒ��ʧ��
��5��ͨ�����㣬����������IJ�����65%��
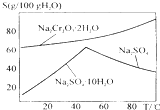 ��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�
��ҵ���Ը�������Ҫ�ɷ�FeO•Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7•2H2O������Ҫ��Ӧ���£�